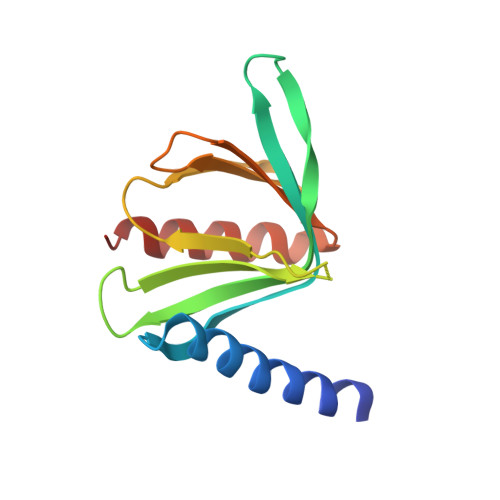
Structural Basis of the Pnrc2-Mediated Link between Mrna Surveillance and Decapping.
Lai, T., Cho, H., Liu, Z., Bowler, M.W., Piao, S., Parker, R., Kim, Y.K., Song, H.(2012) Structure 20: 2025
- PubMed: 23085078
- DOI: https://doi.org/10.1016/j.str.2012.09.009
- Primary Citation of Related Structures:
4B6H - PubMed Abstract:
Nonsense-mediated mRNA decay (NMD) is an important mRNA surveillance system, and human PNRC2 protein mediates the link between mRNA surveillance and decapping. However, the mechanism by which PNRC2 interacts with the mRNA surveillance machinery and stimulates NMD is unknown. Here, we present the crystal structure of Dcp1a in complex with PNRC2. The proline-rich region of PNRC2 is bound to the EVH1 domain of Dcp1a, while its NR-box mediates the interaction with the hyperphosphorylated Upf1. The mode of PNRC2 interaction with Dcp1a is distinct from those observed in other EVH1/proline-rich ligands interactions. Disruption of the interaction of PNRC2 with Dcp1a abolishes its P-body localization and ability to promote mRNA degradation when tethered to mRNAs. PNRC2 acts in synergy with Dcp1a to stimulate the decapping activity of Dcp2 by bridging the interaction between Dcp1a and Dcp2, suggesting that PNRC2 is a decapping coactivator in addition to its adaptor role in NMD.
- Institute of Molecular and Cell Biology, 61 Biopolis Drive, Singapore 138673.
Organizational Affiliation: