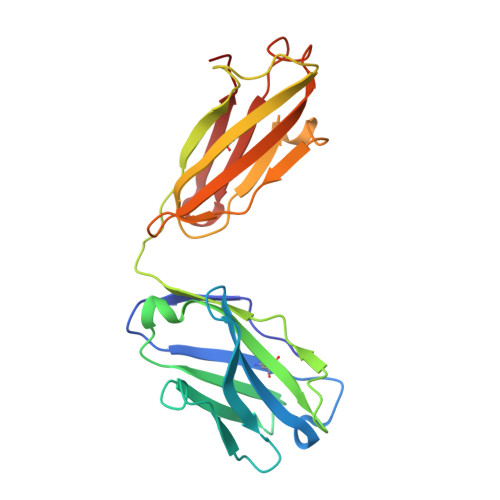
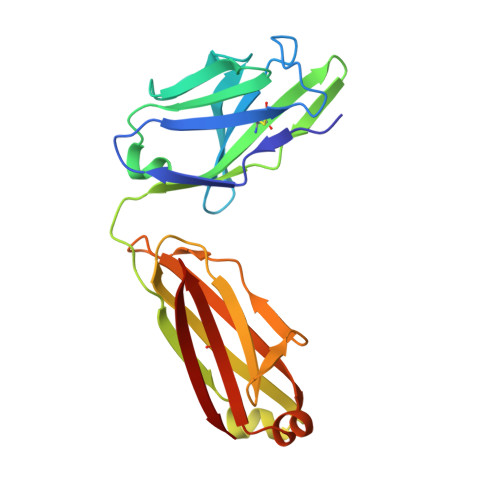
Crystal Structure of Two Anti-Porphyrin Antibodies with Peroxidase Activity.
Munoz Robles, V., Marechal, J., Bahloul, A., Sari, M., Mahy, J., Golinelli-Pimpaneau, B.(2012) PLoS One 7: 51128
- PubMed: 23240001
- DOI: https://doi.org/10.1371/journal.pone.0051128
- Primary Citation of Related Structures:
4AMK, 4AT6 - PubMed Abstract:
We report the crystal structures at 2.05 and 2.45 Å resolution of two antibodies, 13G10 and 14H7, directed against an iron(III)-αααβ-carboxyphenylporphyrin, which display some peroxidase activity. Although these two antibodies differ by only one amino acid in their variable λ-light chain and display 86% sequence identity in their variable heavy chain, their complementary determining regions (CDR) CDRH1 and CDRH3 adopt very different conformations. The presence of Met or Leu residues at positions preceding residue H101 in CDRH3 in 13G10 and 14H7, respectively, yields to shallow combining sites pockets with different shapes that are mainly hydrophobic. The hapten and other carboxyphenyl-derivatized iron(III)-porphyrins have been modeled in the active sites of both antibodies using protein ligand docking with the program GOLD. The hapten is maintained in the antibody pockets of 13G10 and 14H7 by a strong network of hydrogen bonds with two or three carboxylates of the carboxyphenyl substituents of the porphyrin, respectively, as well as numerous stacking and van der Waals interactions with the very hydrophobic CDRH3. However, no amino acid residue was found to chelate the iron. Modeling also allows us to rationalize the recognition of alternative porphyrinic cofactors by the 13G10 and 14H7 antibodies and the effect of imidazole binding on the peroxidase activity of the 13G10/porphyrin complexes.
- Departament de Química, Universitat Autònoma de Barcelona, Edifici C.n., 08193 Cerdanyola del Vallès, Barcelona, Spain.
Organizational Affiliation: