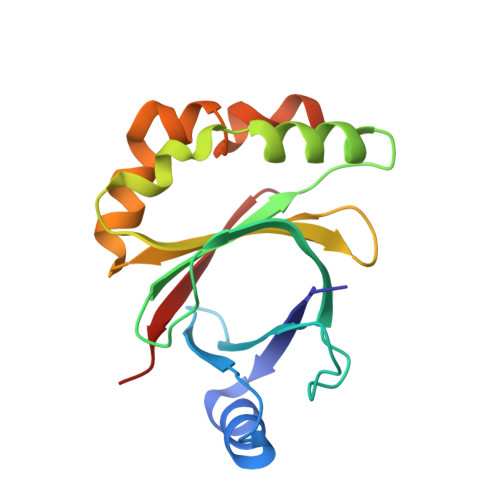
Structure of the Signal Transduction Protein Trap (Target of Rnaiii-Activating Protein).
Henrick, K., Hirshberg, M.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 744
- PubMed: 22750855
- DOI: https://doi.org/10.1107/S1744309112020167
- Primary Citation of Related Structures:
4AE5 - PubMed Abstract:
The crystal structure of the signal transduction protein TRAP is reported at 1.85 Å resolution. The structure of TRAP consists of a central eight-stranded β-barrel flanked asymmetrically by helices and is monomeric both in solution and in the crystal structure. A formate ion was found bound to TRAP identically in all four molecules in the asymmetric unit.
- Research Collaboratory for Structural Bioinformatics Protein Data Bank, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 610 Taylor Road, Piscataway, NJ 08854-8087, USA.
Organizational Affiliation: