Symmetric Dimethylation of H3R2 is a Newly Identified Histone Mark that Supports Euchromatin Maintenance
Migliori, V., Muller, J., Phalke, S., Low, D., Bezzi, M., Chuenmok, W., Sahu, S.K., Gunaratne, J., Capasso, P., Bassi, C., Cecatiello, V., Demarco, A., Blackstock, W., Kuznetsov, V., Amati, B., Mapelli, M., Guccione, E.(2012) Nat Struct Mol Biol 19: 136
- PubMed: 22231400
- DOI: https://doi.org/10.1038/nsmb.2209
- Primary Citation of Related Structures:
4A7J - PubMed Abstract:
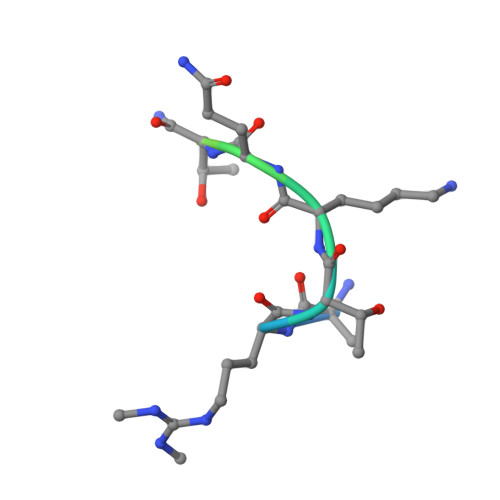
The asymmetric dimethylation of histone H3 arginine 2 (H3R2me2a) acts as a repressive mark that antagonizes trimethylation of H3 lysine 4. Here we report that H3R2 is also symmetrically dimethylated (H3R2me2s) by PRMT5 and PRMT7 and present in euchromatic regions. Profiling of H3-tail interactors by SILAC MS revealed that H3R2me2s excludes binding of RBBP7, a central component of co-repressor complexes Sin3a, NURD and PRC2. Conversely H3R2me2s enhances binding of WDR5, a common component of the coactivator complexes MLL, SET1A, SET1B, NLS1 and ATAC. The interaction of histone H3 with WDR5 distinguishes H3R2me2s from H3R2me2a, which impedes the recruitment of WDR5 to chromatin. The crystallographic structure of WDR5 and the H3R2me2s peptide elucidates the molecular determinants of this high affinity interaction. Our findings identify H3R2me2s as a previously unknown mark that keeps genes poised in euchromatin for transcriptional activation upon cell-cycle withdrawal and differentiation in human cells.
- Institute of Molecular and Cell Biology, Singapore.
Organizational Affiliation: