Catalytic Function and Substrate Recognition of the Pectate Lyase from Thermotoga Maritima
McDonough, M.A., Thymark, M., Frisner, H., Hotchkiss, A., Sonksen, C., Bjornvad, M., Johansen, K.S., Larsen, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PECTATE TRISACCHARIDE-LYASE | 340 | Thermotoga maritima | Mutation(s): 3 EC: 4.2.2.22 | 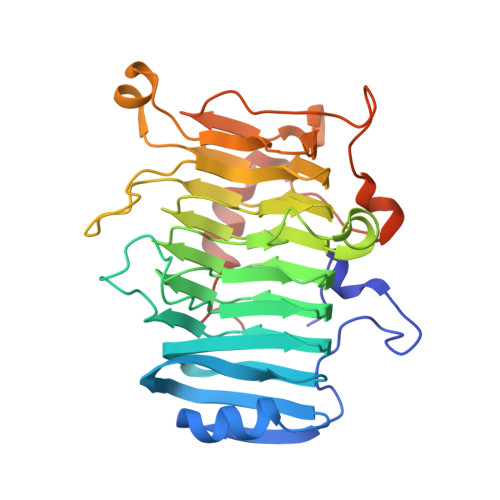 | |
UniProt | |||||
Find proteins for Q9WYR4 (Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8)) Explore Q9WYR4 Go to UniProtKB: Q9WYR4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9WYR4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PO4 Query on PO4 | E [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | C [auth A], D [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 80.602 | α = 90 |
| b = 80.602 | β = 90 |
| c = 148.819 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| EPMR | phasing |