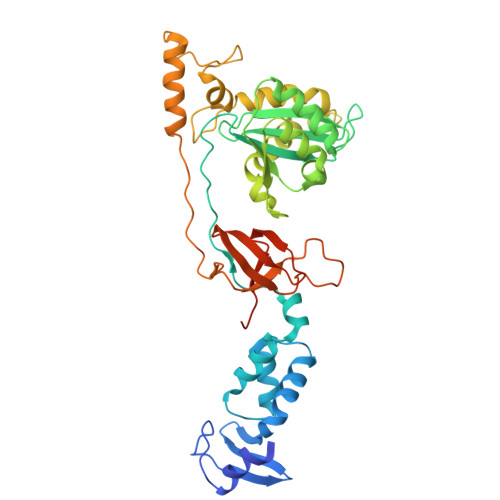
Structural and Functional Analyses of the Second-Generation Integrase Strand Transfer Inhibitor Dolutegravir (S/GSK1349572).
Hare, S., Smith, S.J., Metifiot, M., Jaxa-Chamiec, A., Pommier, Y., Hughes, S.H., Cherepanov, P.(2011) Mol Pharmacol 80: 565-572
- PubMed: 21719464
- DOI: https://doi.org/10.1124/mol.111.073189
- Primary Citation of Related Structures:
3S3M, 3S3N, 3S3O - PubMed Abstract:
Raltegravir (RAL) and related HIV-1 integrase (IN) strand transfer inhibitors (INSTIs) efficiently block viral replication in vitro and suppress viremia in patients. These small molecules bind to the IN active site, causing it to disengage from the deoxyadenosine at the 3' end of viral DNA. The emergence of viral strains that are highly resistant to RAL underscores the pressing need to develop INSTIs with improved resistance profiles. Herein, we show that the candidate second-generation drug dolutegravir (DTG, S/GSK1349572) effectively inhibits a panel of HIV-1 IN variants resistant to first-generation INSTIs. To elucidate the structural basis for the increased potency of DTG against RAL-resistant INs, we determined crystal structures of wild-type and mutant prototype foamy virus intasomes bound to this compound. The overall IN binding mode of DTG is strikingly similar to that of the tricyclic hydroxypyrrole MK-2048. Both second-generation INSTIs occupy almost the same physical space within the IN active site and make contacts with the β4-α2 loop of the catalytic core domain. The extended linker region connecting the metal chelating core and the halobenzyl group of DTG allows it to enter farther into the pocket vacated by the displaced viral DNA base and to make more intimate contacts with viral DNA, compared with those made by RAL and other INSTIs. In addition, our structures suggest that DTG has the ability to subtly readjust its position and conformation in response to structural changes in the active sites of RAL-resistant INs.
- Division of Infectious Diseases, Imperial College London, London, United Kingdom.
Organizational Affiliation: