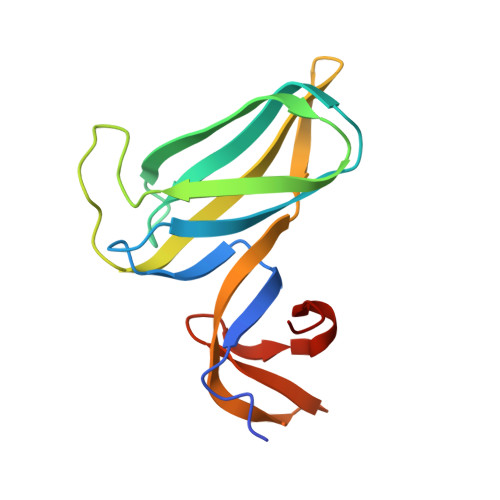
Crystal structure of a novel dimer form of FlgD from P. aeruginosa PAO1
Zhou, H., Luo, M., Cai, X., Tang, J., Niu, S., Zhang, W., Hu, Y., Yin, Y., Huang, A., Wang, D., Wang, D.(2011) Proteins 79: 2346-2351
- PubMed: 21604306
- DOI: https://doi.org/10.1002/prot.23058
- Primary Citation of Related Structures:
3OSV - Key Laboratory of Molecular Biology on Infectious Disease, Chongqing Medical University, YiXueYuanlu-1, Chongqing 400016, China.
Organizational Affiliation: