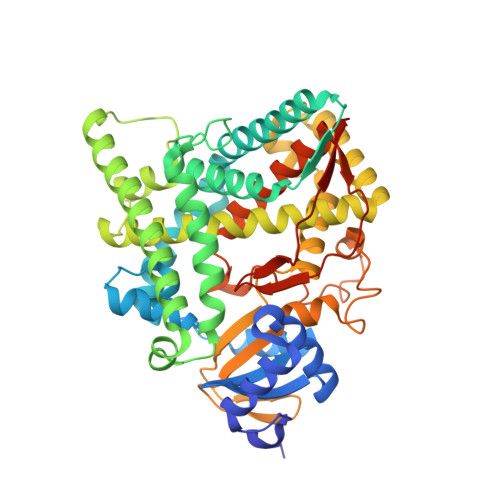
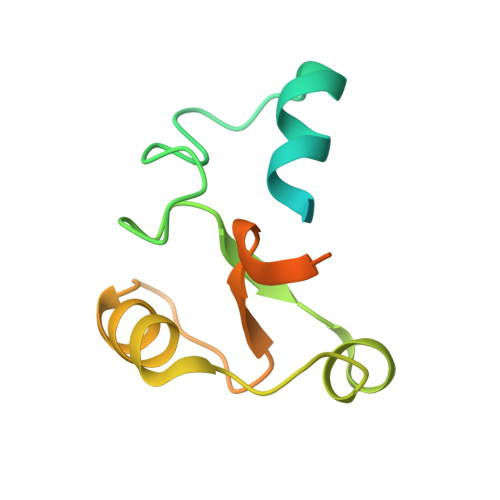
Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system.
Strushkevich, N., Mackenzie, F., Cherkesova, T., Grabovec, I., Usanov, S., Park, H.W.(2011) Proc Natl Acad Sci U S A 108: 10139-10143
- PubMed: 21636783
- DOI: https://doi.org/10.1073/pnas.1019441108
- Primary Citation of Related Structures:
3N9Y, 3N9Z, 3NA0, 3NA1 - PubMed Abstract:
In humans, the precursor to all steroid hormones, pregnenolone, is synthesized from cholesterol by an enzyme complex comprising adrenodoxin reductase (AdR), adrenodoxin (Adx), and a cytochrome P450 (P450scc or CYP11A1). This complex not only plays a key role in steroidogenesis, but also has long been a model to study electron transfer, multistep catalysis, and C-C bond cleavage performed by monooxygenases. Detailed mechanistic understanding of these processes has been hindered by a lack of structural information. Here we present the crystal structure of the complex of human Adx and CYP11A1--the first of a complex between a eukaryotic CYP and its redox partner. The structures with substrate and a series of reaction intermediates allow us to define the mechanism underlying sequential hydroxylations of the cholesterol and suggest the mechanism of C-C bond cleavage. In the complex the [2Fe-2S] cluster of Adx is positioned 17.4 Å away from the heme iron of CYP11A1. This structure suggests that after an initial protein-protein association driven by electrostatic forces, the complex adopts an optimized geometry between the redox centers. Conservation of the interaction interface suggests that this mechanism is common for all mitochondrial P450s.
- Structural Genomics Consortium, University of Toronto, 101 College Street, Toronto, ON, Canada M5G 1L7.
Organizational Affiliation: