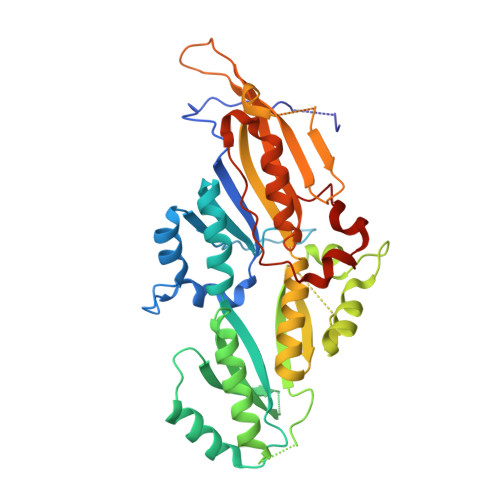
Four KH domains of the C. elegans Bicaudal-C ortholog GLD-3 form a globular structural platform.
Nakel, K., Hartung, S.A., Bonneau, F., Eckmann, C.R., Conti, E.(2010) RNA 16: 2058-2067
- PubMed: 20823118
- DOI: https://doi.org/10.1261/rna.2315010
- Primary Citation of Related Structures:
3N89 - PubMed Abstract:
Caenorhabditis elegans GLD-3 is a five K homology (KH) domain-containing protein involved in the translational control of germline-specific mRNAs during embryogenesis. GLD-3 interacts with the cytoplasmic poly(A)-polymerase GLD-2. The two proteins cooperate to recognize target mRNAs and convert them into a polyadenylated, translationally active state. We report the 2.8-Å-resolution crystal structure of a proteolytically stable fragment encompassing the KH2, KH3, KH4, and KH5 domains of C. elegans GLD-3. The structure reveals that the four tandem KH domains are organized into a globular structural unit. The domains are involved in extensive side-by-side interactions, similar to those observed in previous structures of dimeric KH domains, as well as head-to-toe interactions. Small-angle X-ray scattering reconstructions show that the N-terminal KH domain (KH1) forms a thumb-like protrusion on the KH2-KH5 unit. Although KH domains are putative RNA-binding modules, the KH region of GLD-3 is unable in isolation to cross-link RNA. Instead, the KH1 domain mediates the direct interaction with the poly(A)-polymerase GLD-2, pointing to a function of the KH region as a protein-protein interaction platform.
- Department of Structural Cell Biology, Max-Planck-Institute of Biochemistry, D-82152 Martinsried, Germany.
Organizational Affiliation: