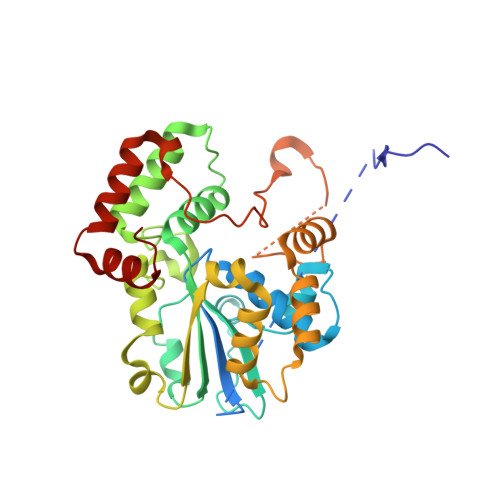
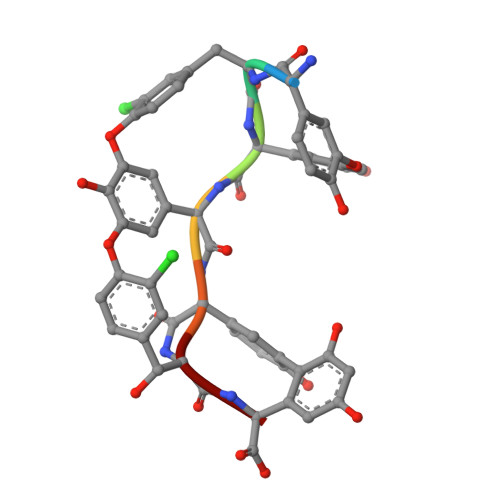
Crystal Structures of the Glycopeptide Sulfotransferase Teg12 in a Complex with the Teicoplanin Aglycone.
Bick, M.J., Banik, J.J., Darst, S.A., Brady, S.F.(2010) Biochemistry 49: 4159
- PubMed: 20361791
- DOI: https://doi.org/10.1021/bi100150v
- Primary Citation of Related Structures:
3MG9, 3MGB, 3MGC - PubMed Abstract:
The TEG gene cluster, a glycopeptide biosynthetic gene cluster that is predicted to encode the biosynthesis of a polysulfated glycopeptide congener, was recently cloned from DNA extracted directly from desert soil. This predicted glycopeptide gene cluster contains three closely related sulfotransferases (Teg12, -13, and -14) that sulfate teicoplanin-like glycopeptides at three unique sites. Here we report a series of structures: an apo structure of Teg12, Teg12 bound to the desulfated cosubstrate 3'-phosphoadenosine 5'-phosphate, and Teg12 bound to the teicoplanin aglycone. Teg12 appears to undergo a series of significant conformational rearrangements during glycopeptide recruitment, binding, and catalysis. Loop regions that exhibit the most conformational flexibility show the least sequence conservation between TEG sulfotransferases. Site-directed mutagenesis guided by our structural studies confirmed the importance of key catalytic residues as well as the importance of residues found throughout the conformationally flexible loop regions.
- Howard Hughes Medical Institute, Laboratory of Genetically Encoded Small Molecules, Laboratory of Molecular Biophysics, The Rockefeller University, 1230 York Avenue, New York, New York 10065, USA.
Organizational Affiliation: