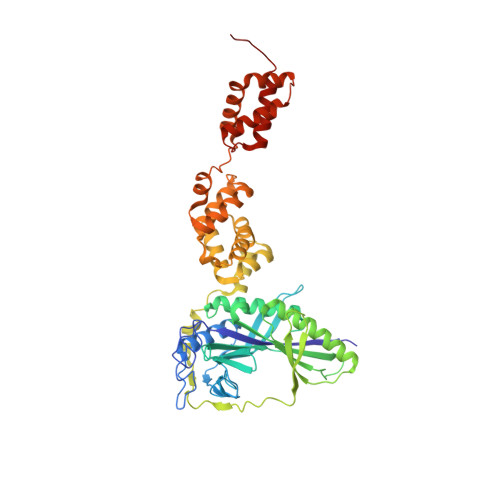
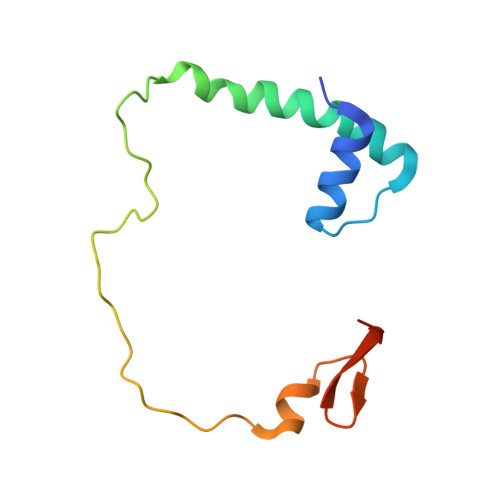
Two distinct regions in Staphylococcus aureus GatCAB guarantee accurate tRNA recognition
Nakamura, A., Sheppard, K., Yamane, J., Yao, M., Soll, D., Tanaka, I.(2010) Nucleic Acids Res 38: 672-682
- PubMed: 19906721
- DOI: https://doi.org/10.1093/nar/gkp955
- Primary Citation of Related Structures:
3IP4 - PubMed Abstract:
In many prokaryotes the biosynthesis of the amide aminoacyl-tRNAs, Gln-tRNA(Gln) and Asn-tRNA(Asn), proceeds by an indirect route in which mischarged Glu-tRNA(Gln) or Asp-tRNA(Asn) is amidated to the correct aminoacyl-tRNA catalyzed by a tRNA-dependent amidotransferase (AdT). Two types of AdTs exist: bacteria, archaea and organelles possess heterotrimeric GatCAB, while heterodimeric GatDE occurs exclusively in archaea. Bacterial GatCAB and GatDE recognize the first base pair of the acceptor stem and the D-loop of their tRNA substrates, while archaeal GatCAB recognizes the tertiary core of the tRNA, but not the first base pair. Here, we present the crystal structure of the full-length Staphylococcus aureus GatCAB. Its GatB tail domain possesses a conserved Lys rich motif that is situated close to the variable loop in a GatCAB:tRNA(Gln) docking model. This motif is also conserved in the tail domain of archaeal GatCAB, suggesting this basic region may recognize the tRNA variable loop to discriminate Asp-tRNA(Asn) from Asp-tRNA(Asp) in archaea. Furthermore, we identified a 3(10) turn in GatB that permits the bacterial GatCAB to distinguish a U1-A72 base pair from a G1-C72 pair; the absence of this element in archaeal GatCAB enables the latter enzyme to recognize aminoacyl-tRNAs with G1-C72 base pairs.
- Division of Biological Sciences, Graduate School of Science, Hokkaido University, Sapporo 060-0810, Japan.
Organizational Affiliation: