Structure of Protein serine/threonine phosphatase from Saccharomyces cerevisiae with similarity to human phosphatase PP5
Singer, A.U., Xu, X., Chang, C., Cui, H., Edwards, A.M., Joachimiak, A., Savchenko, A., Yakunin, A.F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine/threonine-protein phosphatase T | 335 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: G6347, PPT1, YGR123C EC: 3.1.3.16 | 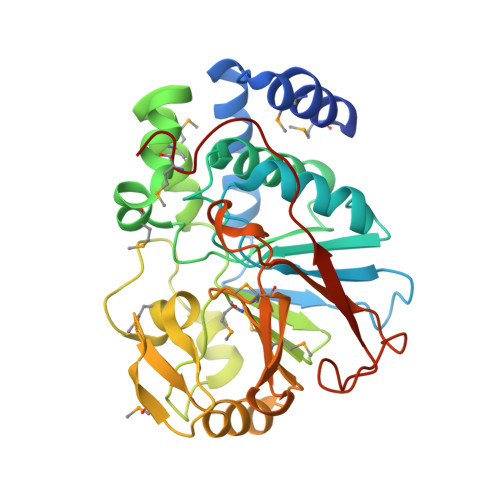 | |
UniProt | |||||
Find proteins for P53043 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P53043 Go to UniProtKB: P53043 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53043 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PO4 Query on PO4 | E [auth A], J [auth B] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| EDO Query on EDO | F [auth A], K [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| FE Query on FE | C [auth A], D [auth A], H [auth B], I [auth B] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| CL Query on CL | L [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| NA Query on NA | G [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.194 | α = 90 |
| b = 47.194 | β = 90 |
| c = 237.096 | γ = 120 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| HKL-3000 | data reduction |
| HKL-2000 | data scaling |