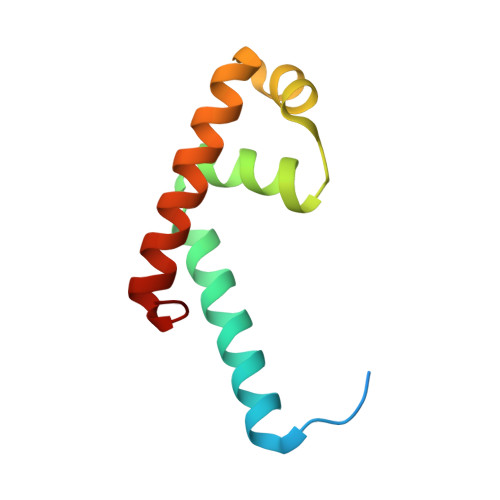
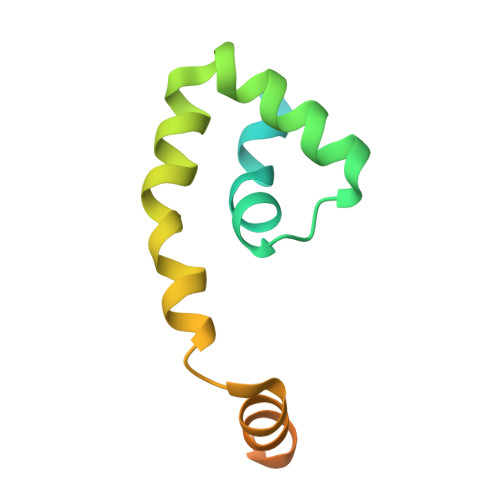
Structural and biochemical bases for the redox sensitivity of Mycobacterium tuberculosis RslA
Thakur, K.G., Praveena, T., Gopal, B.(2010) J Mol Biology 397: 1199-1208
- PubMed: 20184899
- DOI: https://doi.org/10.1016/j.jmb.2010.02.026
- Primary Citation of Related Structures:
3HUG - PubMed Abstract:
An effective transcriptional response to redox stimuli is of particular importance for Mycobacterium tuberculosis, as it adapts to the environment of host alveoli and macrophages. The M. tuberculosis sigma factor sigma(L) regulates the expression of genes involved in cell-wall and polyketide syntheses. sigma(L) interacts with the cytosolic anti-sigma domain of a membrane-associated protein, RslA. Here we demonstrate that RslA binds Zn(2+) and can sequester sigma(L) in a reducing environment. In response to an oxidative stimulus, proximal cysteines in the CXXC motif of RslA form a disulfide bond, releasing bound Zn(2+). This results in a substantial rearrangement of the sigma(L)/RslA complex, leading to an 8-fold decrease in the affinity of RslA for sigma(L). The crystal structure of the -35-element recognition domain of sigma(L), sigma(4)(L), bound to RslA reveals that RslA inactivates sigma(L) by sterically occluding promoter DNA and RNA polymerase binding sites. The crystal structure further reveals that the cysteine residues that coordinate Zn(2+) in RslA are solvent exposed in the complex, thus providing a structural basis for the redox sensitivity of RslA. The biophysical parameters of sigma(L)/RslA interactions provide a template for understanding how variations in the rate of Zn(2+) release and associated conformational changes could regulate the activity of a Zn(2+)-associated anti-sigma factor.
- Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.
Organizational Affiliation: