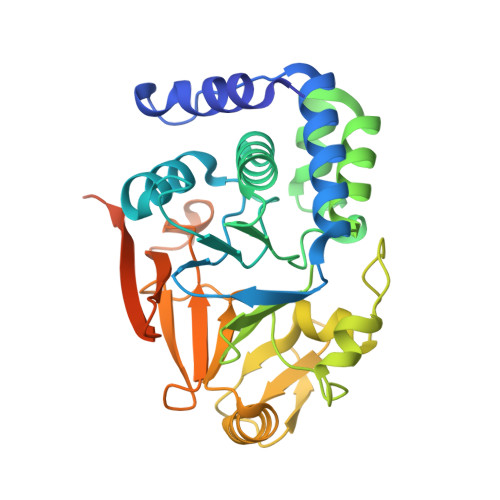
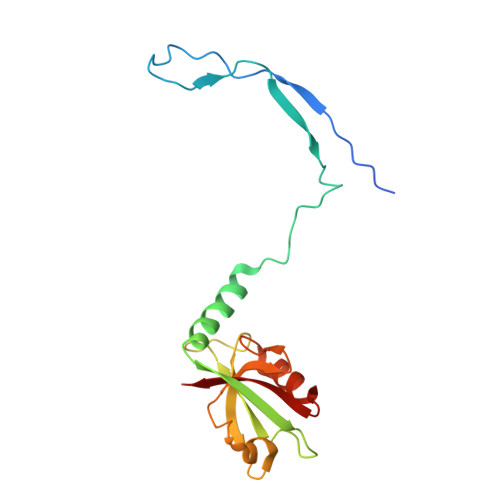
Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites.
Ragusa, M.J., Dancheck, B., Critton, D.A., Nairn, A.C., Page, R., Peti, W.(2010) Nat Struct Mol Biol 17: 459-464
- PubMed: 20305656
- DOI: https://doi.org/10.1038/nsmb.1786
- Primary Citation of Related Structures:
3EGG, 3EGH, 3HVQ - PubMed Abstract:
The serine/threonine protein phosphatase 1 (PP1) dephosphorylates hundreds of key biological targets. PP1 associates with >or=200 regulatory proteins to form highly specific holoenzymes. These regulatory proteins target PP1 to its point of action within the cell and prime its enzymatic specificity for particular substrates. However, how they direct PP1's specificity is not understood. Here we show that spinophilin, a neuronal PP1 regulator, is entirely unstructured in its unbound form, and it binds PP1 through a folding-upon-binding mechanism in an elongated fashion, blocking one of PP1's three putative substrate binding sites without altering its active site. This mode of binding is sufficient for spinophilin to restrict PP1's activity toward a model substrate in vitro without affecting its ability to dephosphorylate its neuronal substrate, glutamate receptor 1 (GluR1). Thus, our work provides the molecular basis for the ability of spinophilin to dictate PP1 substrate specificity.
- Department of Molecular Pharmacology, Physiology and Biotechnology, Brown University, Providence, Rhode Island, USA.
Organizational Affiliation: