Inhibitor design to Ribonuclease A: The binding of two 5'phosphate uridine analogues
Tsirkone, V.G., Dossi, K., Drakou, C., Zographos, S.E., Kontou, M., Leonidas, D.D.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun
Experimental Data Snapshot
Starting Model: experimental
View more details
(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ribonuclease pancreatic | 124 | Bos taurus | Mutation(s): 0 EC: 3.1.27.5 (PDB Primary Data), 4.6.1.18 (UniProt) | 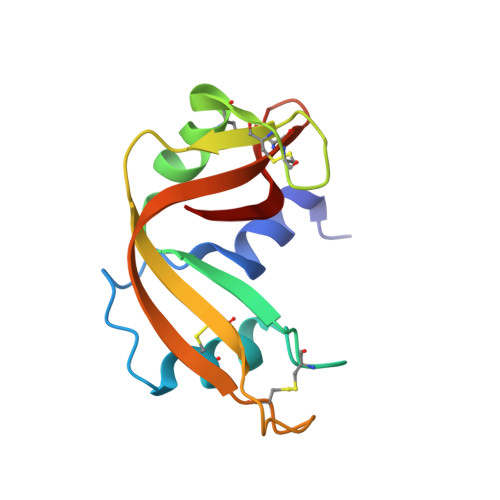 | |
UniProt | |||||
Find proteins for P61823 (Bos taurus) Explore P61823 Go to UniProtKB: P61823 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61823 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| U5P Query on U5P | C [auth A], D [auth A], E [auth B] | URIDINE-5'-MONOPHOSPHATE C9 H13 N2 O9 P DJJCXFVJDGTHFX-XVFCMESISA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 100.035 | α = 90 |
| b = 32.299 | β = 90.91 |
| c = 72.475 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MAR345 | data collection |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| REFMAC | phasing |