Crystal structure of predicted hydrolase of haloacid dehalogenase-like superfamily (NP_295428.1) from Deinococcus radiodurans at 1.66 A resolution
Joint Center for Structural Genomics (JCSG)To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hydrolase family protein | 200 | Deinococcus radiodurans R1 = ATCC 13939 = DSM 20539 | Mutation(s): 0 Gene Names: NP_295428.1, DR_1705 | 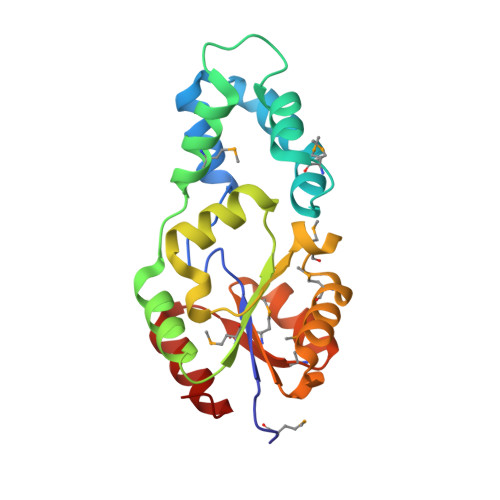 | |
UniProt | |||||
Find proteins for Q9RTQ1 (Deinococcus radiodurans (strain ATCC 13939 / DSM 20539 / JCM 16871 / CCUG 27074 / LMG 4051 / NBRC 15346 / NCIMB 9279 / VKM B-1422 / R1)) Explore Q9RTQ1 Go to UniProtKB: Q9RTQ1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9RTQ1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PG4 Query on PG4 | E [auth A] | TETRAETHYLENE GLYCOL C8 H18 O5 UWHCKJMYHZGTIT-UHFFFAOYSA-N |  | ||
| PO4 Query on PO4 | D [auth A], G [auth B] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | H [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| NA Query on NA | C [auth A], F [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 74.108 | α = 90 |
| b = 74.108 | β = 90 |
| c = 160.923 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PHENIX | refinement |
| SHELX | phasing |
| MolProbity | model building |
| XSCALE | data scaling |
| PDB_EXTRACT | data extraction |
| MAR345 | data collection |
| XDS | data reduction |
| SHELXD | phasing |
| autoSHARP | phasing |