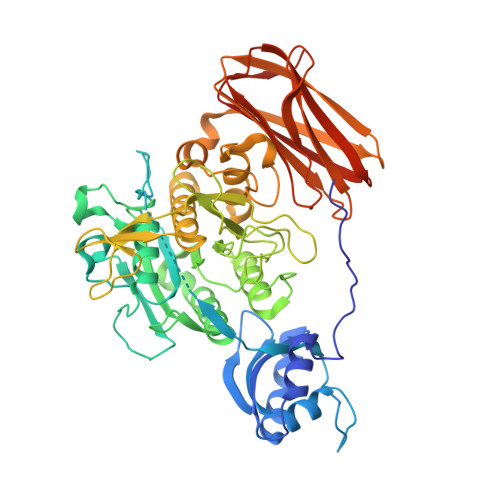
Crystal structure of a subtilisin homologue, Tk-SP, from Thermococcus kodakaraensis: requirement of a C-terminal beta-jelly roll domain for hyperstability.
Foophow, T., Tanaka, S., Angkawidjaja, C., Koga, Y., Takano, K., Kanaya, S.(2010) J Mol Biology 400: 865-877
- PubMed: 20595040
- DOI: https://doi.org/10.1016/j.jmb.2010.05.064
- Primary Citation of Related Structures:
3AFG - PubMed Abstract:
Tk-SP is a hyperthermostable subtilisin-like serine protease from Thermococcus kodakaraensis and is autoprocessed from its precursor (Pro-Tk-SP) with N- and C-propeptides. The crystal structure of the active-site mutant of Pro-Tk-SP lacking C-propeptide, ProN-Tk-S359A, was determined at 2.0 A resolution. ProN-Tk-S359A consists of the N-propeptide, subtilisin, and beta-jelly roll domains. Two Ca(2+) ions bind to the beta-jelly roll domain. The overall structure of ProN-Tk-S359A without the beta-jelly roll domain is similar to that of the bacterial propeptide:subtilisin complex, except that it does not contain Ca(2+) ions. To analyze the role of the beta-jelly roll domain of Tk-SP, we constructed a series of the active-site mutants of Tk-SP with (Tk-S359A/C) and without (Tk-S359A/CDeltaJ) beta-jelly roll domain. Both Tk-S359C and Tk-S359CDeltaJ exhibited protease activities in gel assay, indicating that the beta-jelly roll domain is not required for folding or activity. However, the T(m) value of Tk-S359ADeltaJ determined by far-UV CD spectroscopy in the presence of 10-mM CaCl(2) was lower than that of Tk-S359A by 29.4 degrees C. The T(m) value of Tk-S359A was decreased by 29.5 degrees C by the treatment with 10 mM ethylenediaminetetraacetic acid, indicating that the beta-jelly roll domain contributes to the stabilization of Tk-S359A only in a Ca(2+)-bound form. Tk-SP highly resembles subtilisin-like serine proteases from Pyrococcus furiosus, Thermococcus gammatolerans, and Thermococcus onnurineus in size and amino acid sequence. We propose that attachment of a beta-jelly roll domain to the C-terminus is one of the strategies of the proteins from hyperthermophiles to adapt to high-temperature environment.
- Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan.
Organizational Affiliation: