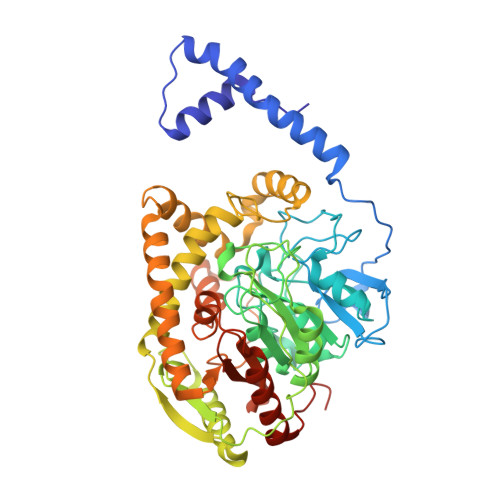
Structure and Characterization of Amidase from Rhodococcus sp. N-771: Insight into the Molecular Mechanism of Substrate Recognition
Ohtaki, A., Murata, K., Sato, Y., Noguchi, K., Miyatake, H., Dohmae, N., Yamada, K., Yohda, M., Odaka, M.(2009) Biochim Biophys Acta
- PubMed: 19819352
- DOI: https://doi.org/10.1016/j.bbapap.2009.10.001
- Primary Citation of Related Structures:
3A1I, 3A1K - PubMed Abstract:
In this study, we have structurally characterized the amidase of a nitrile-degrading bacterium, Rhodococcus sp. N-771 (RhAmidase). RhAmidase belongs to amidase signature (AS) family, a group of amidase families, and is responsible for the degradation of amides produced from nitriles by nitrile hydratase. Recombinant RhAmidase exists as a dimer of about 107 kDa. RhAmidase can hydrolyze acetamide, propionamide, acrylamide and benzamide with kcat/Km values of 1.14+/-0.23 mM(-1)s(-1), 4.54+/-0.09 mM(-1)s(-1), 0.087+/-0.02 mM(-1)s(-1) and 153.5+/-7.1 mM(-1)s(-1), respectively. The crystal structures of RhAmidase and its inactive mutant complex with benzamide (S195A/benzamide) were determined at resolutions of 2.17 A and 2.32 A, respectively. RhAmidase has three domains: an N-terminal alpha-helical domain, a small domain and a large domain. The N-terminal alpha-helical domain is not found in other AS family enzymes. This domain is involved in the formation of the dimer structure and, together with the small domain, forms a narrow substrate-binding tunnel. The large domain showed high structural similarities to those of other AS family enzymes. The Ser-cis Ser-Lys catalytic triad is located in the large domain. But the substrate-binding pocket of RhAmidase is relatively narrow, due to the presence of the helix alpha13 in the small domain. The hydrophobic residues from the small domain are involved in recognizing the substrate. The small domain likely participates in substrate recognition and is related to the difference of substrate specificities among the AS family amidases.
- Department of Biotechnology and Life Science, Tokyo University of Agriculture and Technology, Koganei, Tokyo 184-8588, Japan.
Organizational Affiliation: