A novel allosteric mechanism on protein-DNA interactions underlying the phosphorylation-dependent regulation of Ets1 target gene expressions.
Shiina, M., Hamada, K., Inoue-Bungo, T., Shimamura, M., Uchiyama, A., Baba, S., Sato, K., Yamamoto, M., Ogata, K.(2015) J Mol Biology 427: 1655-1669
- PubMed: 25083921
- DOI: https://doi.org/10.1016/j.jmb.2014.07.020
- Primary Citation of Related Structures:
3WTS, 3WTT, 3WTU, 3WTV, 3WTW, 3WTX, 3WTY, 3WTZ, 3WU0, 3WU1 - PubMed Abstract:
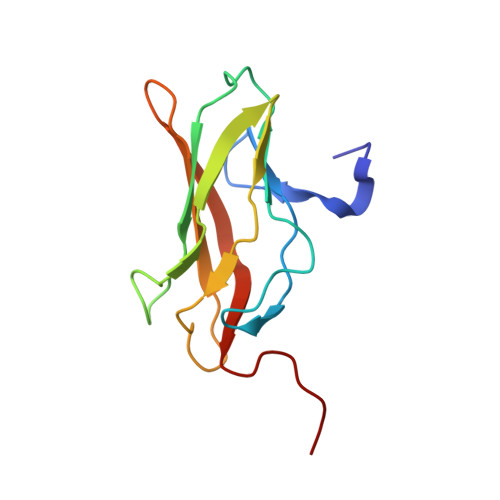
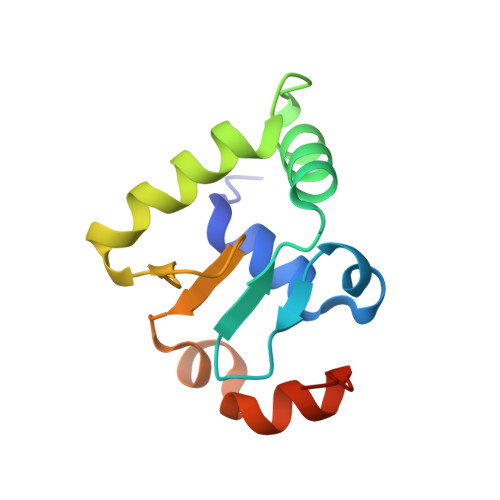
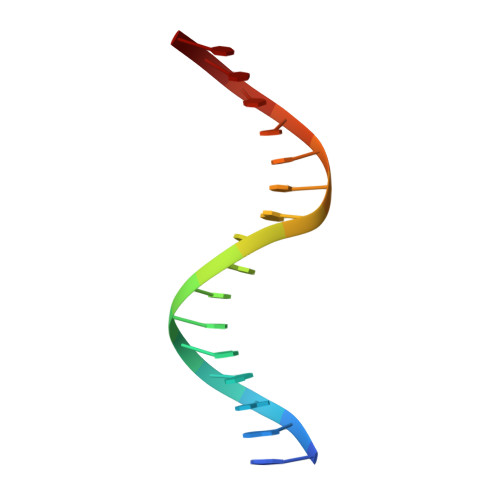
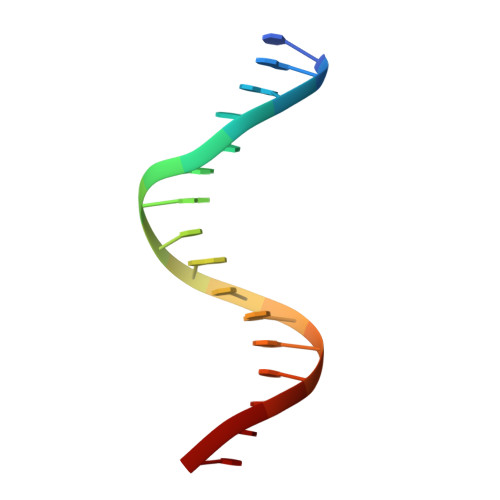
Cooperative assemblies of transcription factors (TFs) on target gene enhancers coordinate cell proliferation, fate specification, and differentiation through precise and complicated transcriptional mechanisms. Chemical modifications, such as phosphorylation, of TFs induced by cell signaling further modulate the dynamic cooperativity of TFs. In this study, we found that various Ets1-containing TF-DNA complexes respond differently to calcium-induced phosphorylation of Ets1, which is known to inhibit Ets1-DNA binding. Crystallographic analysis of a complex comprising Ets1, Runx1, and CBFβ at the TCRα enhancer revealed that Ets1 acquires robust binding stability in the Runx1 and DNA-complexed state, via allosteric mechanisms. This allows phosphorylated Ets1 to be retained at the TCRα enhancer with Runx1, in contrast to other Ets1 target gene enhancers including mb-1 and stromelysin-1. This study provides a structure-based model for cell-signaling-dependent regulation of target genes, mediated via chemical modification of TFs.
- Department of Biochemistry, Yokohama City University Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan; RIKEN SPring-8 Center, 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5148, Japan.
Organizational Affiliation: