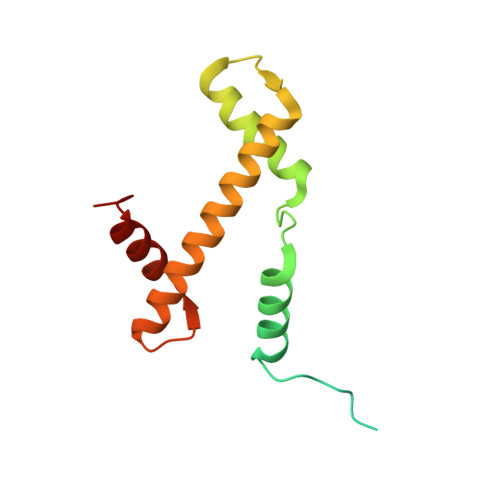
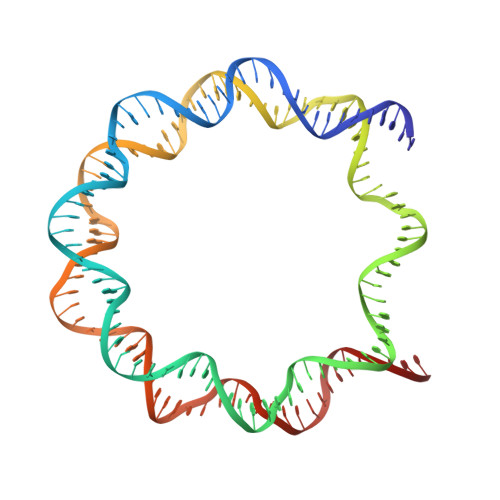
Crystal structure and stable property of the cancer-associated heterotypic nucleosome containing CENP-A and H3.3
Arimura, Y., Shirayama, K., Horikoshi, N., Fujita, R., Taguchi, H., Kagawa, W., Fukagawa, T., Almouzni, G., Kurumizaka, H.(2014) Sci Rep 4: 7115-7115
- PubMed: 25408271
- DOI: https://doi.org/10.1038/srep07115
- Primary Citation of Related Structures:
3WTP - PubMed Abstract:
The centromere-specific histone H3 variant, CENP-A, is overexpressed in particular aggressive cancer cells, where it can be mislocalized ectopically in the form of heterotypic nucleosomes containing H3.3. In the present study, we report the crystal structure of the heterotypic CENP-A/H3.3 particle and reveal its "hybrid structure", in which the physical characteristics of CENP-A and H3.3 are conserved independently within the same particle. The CENP-A/H3.3 nucleosome forms an unexpectedly stable structure as compared to the CENP-A nucleosome, and allows the binding of the essential centromeric protein, CENP-C, which is ectopically mislocalized in the chromosomes of CENP-A overexpressing cells.
- Laboratory of Structural Biology, Graduate School of Advanced Science and Engineering, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan.
Organizational Affiliation: