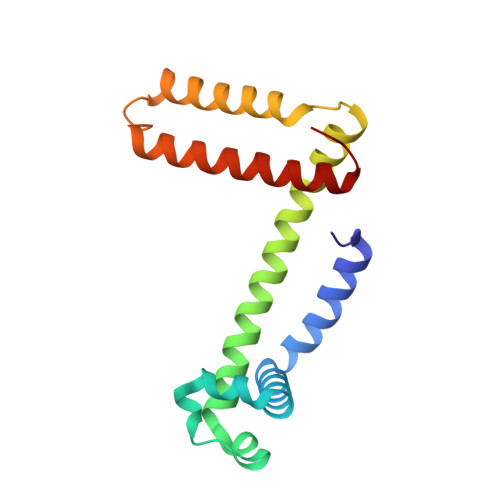
Structural and oxygen binding properties of dimeric horse myoglobin
Nagao, S., Osuka, H., Yamada, T., Uni, T., Shomura, Y., Imai, K., Higuchi, Y., Hirota, S.(2012) Dalton Trans 41: 11378-11385
- PubMed: 22885714
- DOI: https://doi.org/10.1039/c2dt30893b
- Primary Citation of Related Structures:
3VM9 - PubMed Abstract:
Myoglobin (Mb) stores dioxygen in muscles, and is a fundamental model protein widely used in molecular design. The presence of dimeric Mb has been known for more than forty years, but its structural and oxygen binding properties remain unknown. From an X-ray crystallographic analysis at 1.05 Å resolution, we found that dimeric metMb exhibits a domain-swapped structure with two extended α-helices. Each new long α-helix is formed by the E and F helices and the EF-loop of the original monomer, and as a result the proximal and distal histidines of the heme originate from different protomers. The heme orientation in the dimer was in the normal mode as in the monomer, but regulated faster from the reverse to normal orientation. The dimer possessed the oxygen binding property, although it exhibited a slightly higher oxygen binding affinity (∼1.4 fold) compared to the monomer and showed no cooperativity for oxygen binding. The oxygen binding rate constant (k(on)) of the dimer ((14.0 ± 0.7) × 10(6) M(-1) s(-1)) was similar to that of the monomer, whereas the oxygen dissociation rate constant (k(off)) of the dimer (8 ± 1 s(-1)) was smaller than that of the monomer (12 ± 1 s(-1)). We attribute the similar k(on) values to their active site structures being similar, whereas the faster regulation of the heme orientation and the smaller k(off) in the dimer are presumably due to the slight change in the active site structure and/or more rigid structure compared to the monomer. These results show that domain swapping may be a new tool for protein engineering.
- Graduate School of Materials Science, Nara Institute of Science and Technology, 8916-5, Takayama, Ikoma, Nara 630-0192, Japan.
Organizational Affiliation: