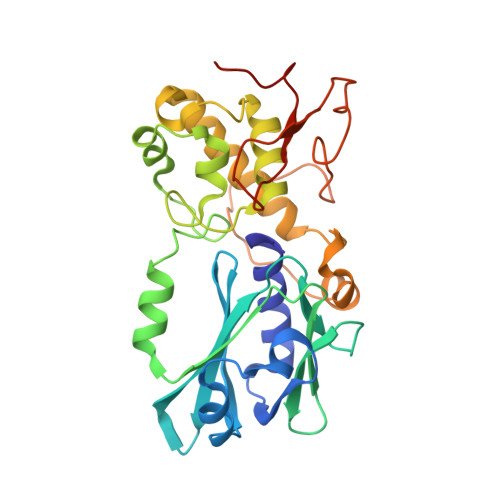
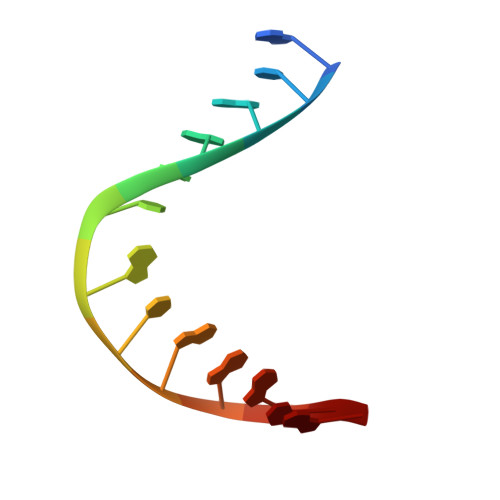
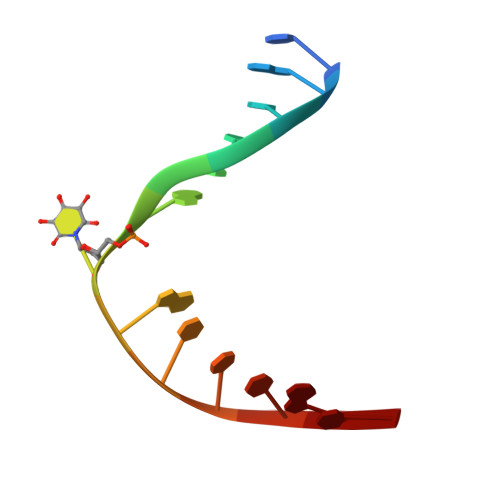
Structural characterization of viral ortholog of human DNA glycosylase NEIL1 bound to thymine glycol or 5-hydroxyuracil-containing DNA
Imamura, K., Averill, A., Wallace, S.S., Doublie, S.(2012) J Biological Chem 287: 4288-4298
- PubMed: 22170059
- DOI: https://doi.org/10.1074/jbc.M111.315309
- Primary Citation of Related Structures:
3VK7, 3VK8 - PubMed Abstract:
Thymine glycol (Tg) and 5-hydroxyuracil (5-OHU) are common oxidized products of pyrimidines, which are recognized and cleaved by two DNA glycosylases of the base excision repair pathway, endonuclease III (Nth) and endonuclease VIII (Nei). Although there are several structures of Nei enzymes unliganded or bound to an abasic (apurinic or apyrimidinic) site, until now there was no structure of an Nei bound to a DNA lesion. Mimivirus Nei1 (MvNei1) is an ortholog of human NEIL1, which was previously crystallized bound to DNA containing an apurinic site (Imamura, K., Wallace, S. S., and Doublié, S. (2009) J. Biol. Chem. 284, 26174-26183). Here, we present two crystal structures of MvNei1 bound to two oxidized pyrimidines, Tg and 5-OHU. Both lesions are flipped out from the DNA helix. Tg is in the anti conformation, whereas 5-OHU adopts both anti and syn conformations in the glycosylase active site. Only two protein side chains (Glu-6 and Tyr-253) are within hydrogen-bonding contact with either damaged base, and mutating these residues did not markedly affect the glycosylase activity. This finding suggests that lesion recognition by Nei occurs before the damaged base flips into the glycosylase active site.
- Department of Microbiology and Molecular Genetics, University of Vermont, Burlington, Vermont 05405, USA.
Organizational Affiliation: