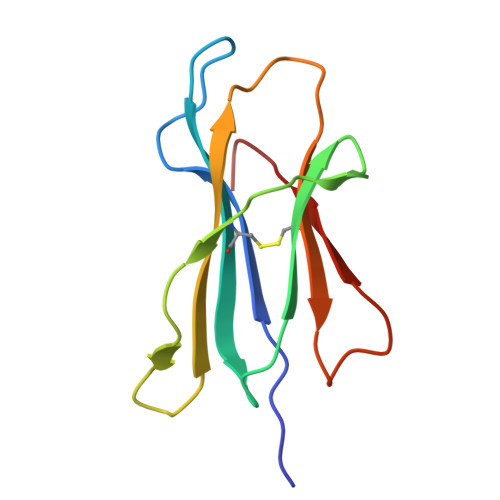
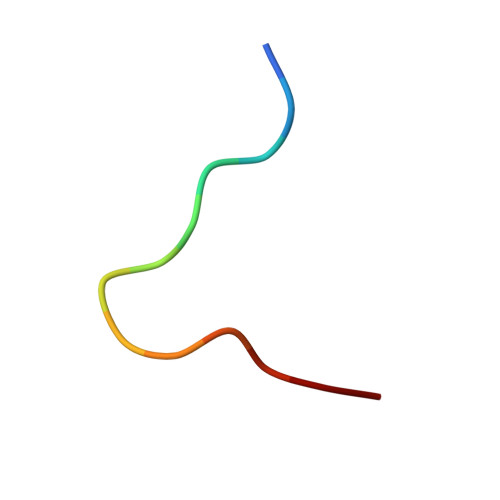
The Energetic Basis Underpinning T-cell Receptor Recognition of a Super-bulged Peptide Bound to a Major Histocompatibility Complex Class I Molecule.
Liu, Y.C., Chen, Z., Burrows, S.R., Purcell, A.W., McCluskey, J., Rossjohn, J., Gras, S.(2012) J Biological Chem 287: 12267-12276
- PubMed: 22343629
- DOI: https://doi.org/10.1074/jbc.M112.344689
- Primary Citation of Related Structures:
3VFM, 3VFN, 3VFO, 3VFP, 3VFR, 3VFS, 3VFT, 3VFU, 3VFV, 3VFW - PubMed Abstract:
Although the major histocompatibility complex class I (MHC-I) molecules typically bind short peptide (p) fragments (8-10 amino acids in length), longer, "bulged" peptides are often be presented by MHC-I. Such bulged pMHC-I complexes represent challenges for T-cell receptor (TCR) ligation, although the general principles underscoring the interaction between TCRs and bulged pMHC-I complexes are unclear. To address this, we have explored the energetic basis of how an immunodominant TCR (termed SB27) binds to a 13-amino acid viral peptide (LPEPLPQGQLTAY) complexed to human leukocyte antigen (HLA) B*3508. Using the crystal structure of the SB27 TCR-HLA B*3508(LPEP) complex as a guide, we undertook a comprehensive alanine-scanning mutagenesis approach at the TCR-pMHC-I interface and examined the effect of the mutations by biophysical (affinity measurements) and cellular approaches (tetramer staining). Although the structural footprint on HLA B*3508 was small, the energetic footprint was even smaller in that only two HLA B*3508 residues were critical for the TCR interaction. Instead, the energetic basis of this TCR-pMHC-I interaction was attributed to peptide-mediated interactions in which the complementarity determining region 3α and germline-encoded complementarity determining region 1β loops of the SB27 TCR played the principal role. Our findings highlight the peptide-centricity of TCR ligation toward a bulged pMHC-I complex.
- Department of Biochemistry and Molecular Biology, Monash University, Clayton, 3800, Australia.
Organizational Affiliation: