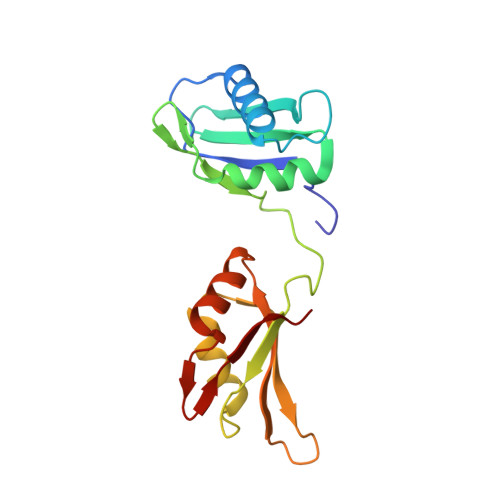
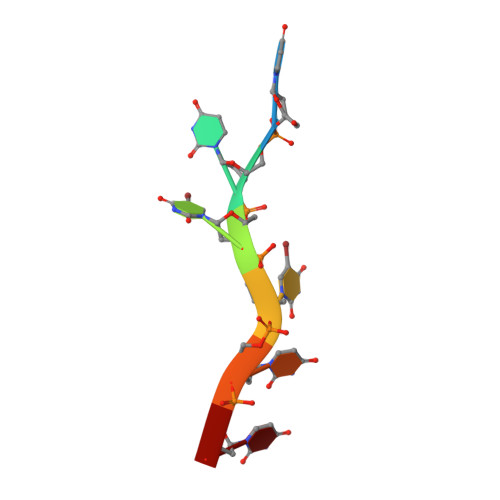
U2AF65 adapts to diverse pre-mRNA splice sites through conformational selection of specific and promiscuous RNA recognition motifs.
Jenkins, J.L., Agrawal, A.A., Gupta, A., Green, M.R., Kielkopf, C.L.(2013) Nucleic Acids Res 41: 3859-3873
- PubMed: 23376934
- DOI: https://doi.org/10.1093/nar/gkt046
- Primary Citation of Related Structures:
3VAF, 3VAG, 3VAH, 3VAI, 3VAJ, 3VAK, 3VAL, 3VAM - PubMed Abstract:
Degenerate splice site sequences mark the intron boundaries of pre-mRNA transcripts in multicellular eukaryotes. The essential pre-mRNA splicing factor U2AF(65) is faced with the paradoxical tasks of accurately targeting polypyrimidine (Py) tracts preceding 3' splice sites while adapting to both cytidine and uridine nucleotides with nearly equivalent frequencies. To understand how U2AF(65) recognizes degenerate Py tracts, we determined six crystal structures of human U2AF(65) bound to cytidine-containing Py tracts. As deoxy-ribose backbones were required for co-crystallization with these Py tracts, we also determined two baseline structures of U2AF(65) bound to the deoxy-uridine counterparts and compared the original, RNA-bound structure. Local structural changes suggest that the N-terminal RNA recognition motif 1 (RRM1) is more promiscuous for cytosine-containing Py tracts than the C-terminal RRM2. These structural differences between the RRMs were reinforced by the specificities of wild-type and site-directed mutant U2AF(65) for region-dependent cytosine- and uracil-containing RNA sites. Small-angle X-ray scattering analyses further demonstrated that Py tract variations select distinct inter-RRM spacings from a pre-existing ensemble of U2AF(65) conformations. Our results highlight both local and global conformational selection as a means for universal 3' splice site recognition by U2AF(65).
- Center for RNA Biology and Department of Biochemistry and Biophysics, University of Rochester School of Medicine and Dentistry, Rochester, NY 14642, USA.
Organizational Affiliation: