Discovery of diarylhydantoins as new selective androgen receptor modulators.
Nique, F., Hebbe, S., Peixoto, C., Annoot, D., Lefrancois, J.-M., Duval, E., Michoux, L., Triballeau, N., Lemoullec, J.M., Mollat, P., Thauvin, M., Prange, T., Minet, D., Clement-Lacroix, P., Robin-Jagerschmidt, C., Fleury, D., Guedin, D., Deprez, P.(2012) J Med Chem 55: 8225-8235
- PubMed: 22897611
- DOI: https://doi.org/10.1021/jm300249m
- Primary Citation of Related Structures:
3V49, 3V4A - PubMed Abstract:
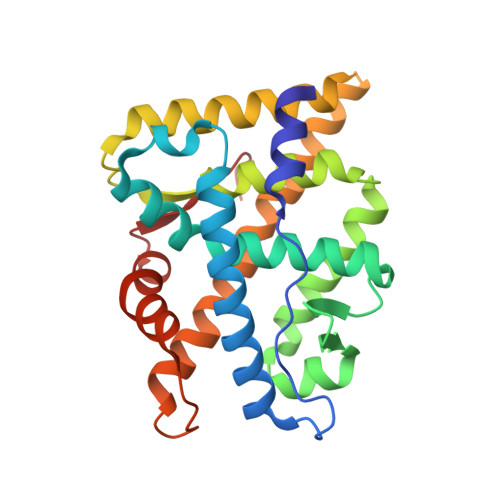
A novel selective androgen receptor modulator scaffold has been discovered through structural modifications of hydantoin antiandrogens. Several 4-(4-hydroxyphenyl)-N-arylhydantoins displayed partial agonism with nanomolar in vitro potency in transactivation experiments using androgen receptor (AR) transfected cells. In a standard castrated male rat model, several compounds showed good anabolic activity on levator ani muscle, dissociated from the androgenic activity on ventral prostate, after oral dosing at 30 mg/kg. (+)-4-[3,4-Dimethyl-2,5-dioxo-4-(4-hydroxyphenyl)imidazolidin-1-yl]-2-(trifluoromethyl)benzonitrile ((+)-11b) displayed anabolic potency with a strong dissociation between levator ani muscle and ventral prostate (A(50) = 0.5 mg/kg vs 70 mg/kg). The binding modes of two compounds, including (+)-11b, within the AR ligand-binding domain have been studied by cocrystallization experiments using a coactivator-like peptide. Both compounds bound to the same site, and the overall structures of the AR were very similar.
- GALAPAGOS, Parc Biocitech, 102 Avenue Gaston Roussel, 93230 Romainville, France.
Organizational Affiliation: