Human caspase 9 in complex with bacterial effector protein
Moertl, M., Maskos, K., Steuber, H.(2013) PLoS One
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2013) PLoS One
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Caspase-9 | 276 | Homo sapiens | Mutation(s): 0 Gene Names: CASP9, MCH6 EC: 3.4.22.62 | 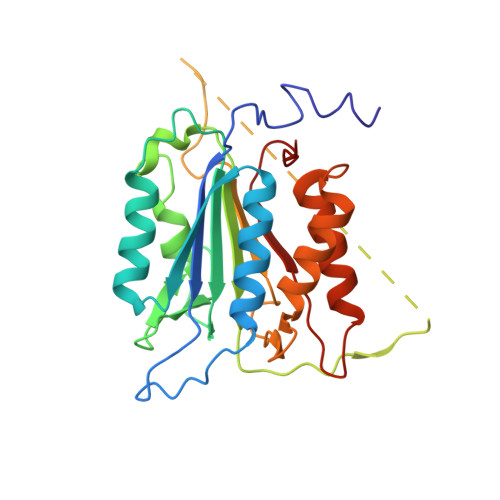 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P55211 (Homo sapiens) Explore P55211 Go to UniProtKB: P55211 | |||||
PHAROS: P55211 GTEx: ENSG00000132906 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P55211 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Putative uncharacterized protein ECs1815 | 165 | Escherichia coli O157:H7 | Mutation(s): 0 Gene Names: ECs1815, Z6020 | 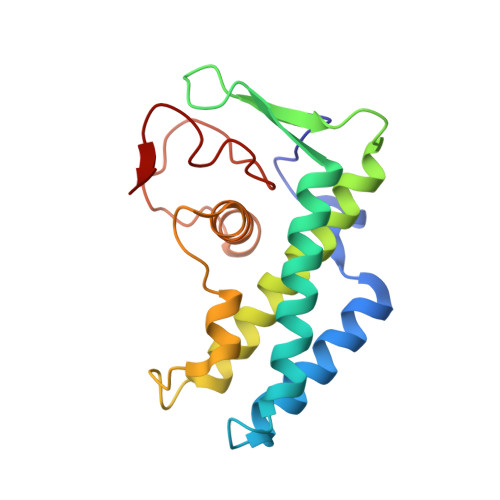 | |
UniProt | |||||
Find proteins for Q8XAL7 (Escherichia coli O157:H7) Explore Q8XAL7 Go to UniProtKB: Q8XAL7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8XAL7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 100.703 | α = 90 |
| b = 209.906 | β = 90 |
| c = 317.231 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MOLREP | phasing |
| REFMAC | refinement |
| XDS | data reduction |
| SCALA | data scaling |