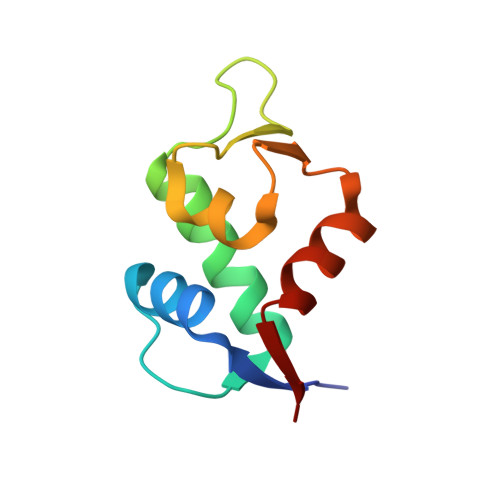
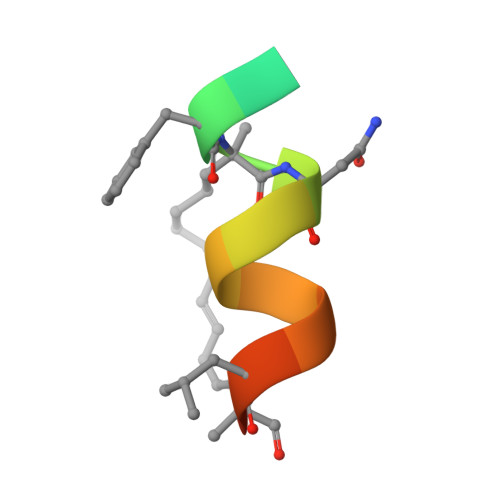
Structure of the stapled p53 peptide bound to Mdm2.
Baek, S., Kutchukian, P.S., Verdine, G.L., Huber, R., Holak, T.A., Lee, K.W., Popowicz, G.M.(2012) J Am Chem Soc 134: 103-106
- PubMed: 22148351
- DOI: https://doi.org/10.1021/ja2090367
- Primary Citation of Related Structures:
3V3B - PubMed Abstract:
Mdm2 is a major negative regulator of the tumor suppressor p53 protein, a protein that plays a crucial role in maintaining genome integrity. Inactivation of p53 is the most prevalent defect in human cancers. Inhibitors of the Mdm2-p53 interaction that restore the functional p53 constitute potential nongenotoxic anticancer agents with a novel mode of action. We present here a 2.0 Å resolution structure of the Mdm2 protein with a bound stapled p53 peptide. Such peptides, which are conformationally and proteolytically stabilized with all-hydrocarbon staples, are an emerging class of biologics that are capable of disrupting protein-protein interactions and thus have broad therapeutic potential. The structure represents the first crystal structure of an i, i + 7 stapled peptide bound to its target and reveals that rather than acting solely as a passive conformational brace, a staple can intimately interact with the surface of a protein and augment the binding interface.
- Max Planck Institute for Biochemistry, Am Klopferspitz 18, 82152 Martinsried, Germany.
Organizational Affiliation: