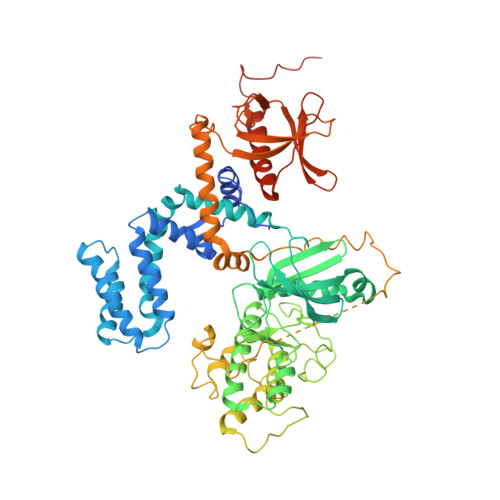
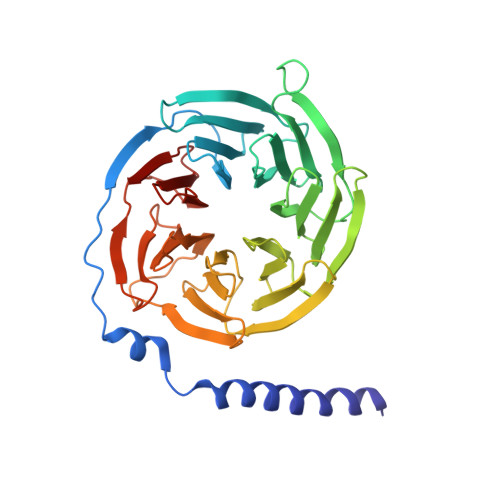
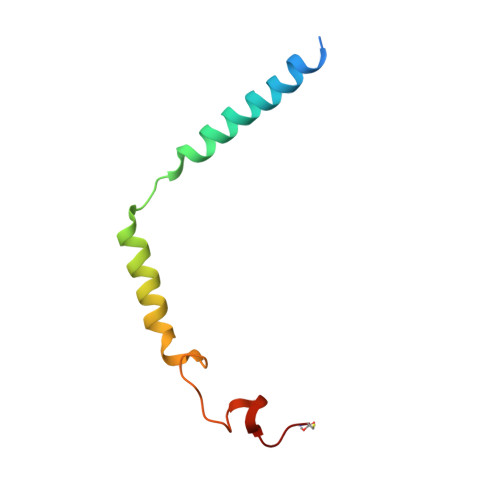
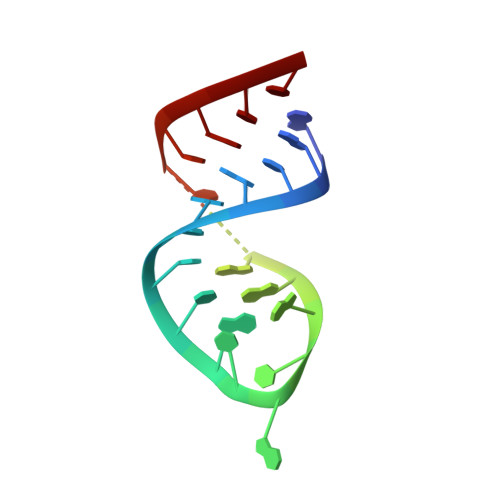
Molecular mechanism for inhibition of g protein-coupled receptor kinase 2 by a selective RNA aptamer.
Tesmer, V.M., Lennarz, S., Mayer, G., Tesmer, J.J.(2012) Structure 20: 1300-1309
- PubMed: 22727813
- DOI: https://doi.org/10.1016/j.str.2012.05.002
- Primary Citation of Related Structures:
3UZS, 3UZT - PubMed Abstract:
Cardiovascular homeostasis is maintained in part by the rapid desensitization of activated heptahelical receptors that have been phosphorylated by G protein-coupled receptor kinase 2 (GRK2). However, during chronic heart failure GRK2 is upregulated and believed to contribute to disease progression. We have determined crystallographic structures of GRK2 bound to an RNA aptamer that potently and selectively inhibits kinase activity. Key to the mechanism of inhibition is the positioning of an adenine nucleotide into the ATP-binding pocket and interactions with the basic αF-αG loop region of the GRK2 kinase domain. Constraints imposed on the RNA by the terminal stem of the aptamer also play a role. These results highlight how a high-affinity aptamer can be used to selectively trap a novel conformational state of a protein kinase.
- Life Sciences Institute, University of Michigan, 210 Washtenaw Avenue, Ann Arbor, MI 48109-2216, USA. tesmerjj@umich.edu
Organizational Affiliation: