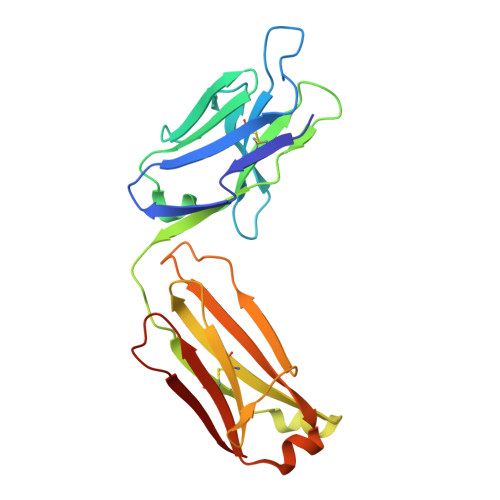
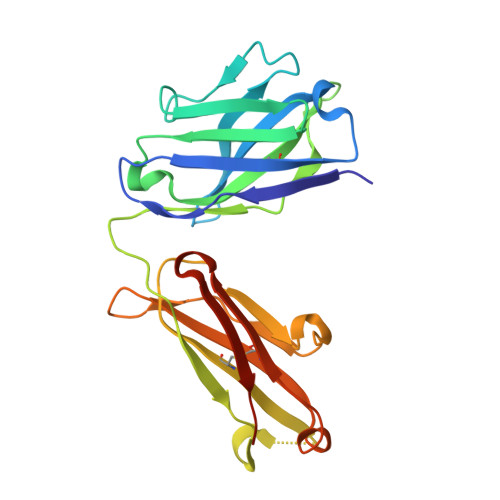
Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential.
Sela-Passwell, N., Kikkeri, R., Dym, O., Rozenberg, H., Margalit, R., Arad-Yellin, R., Eisenstein, M., Brenner, O., Shoham, T., Danon, T., Shanzer, A., Sagi, I.(2012) Nat Med 18: 143-147
- PubMed: 22198278
- DOI: https://doi.org/10.1038/nm.2582
- Primary Citation of Related Structures:
3UTZ - PubMed Abstract:
Endogenous tissue inhibitors of metalloproteinases (TIMPs) have key roles in regulating physiological and pathological cellular processes. Imitating the inhibitory molecular mechanisms of TIMPs while increasing selectivity has been a challenging but desired approach for antibody-based therapy. TIMPs use hybrid protein-protein interactions to form an energetic bond with the catalytic metal ion, as well as with enzyme surface residues. We used an innovative immunization strategy that exploits aspects of molecular mimicry to produce inhibitory antibodies that show TIMP-like binding mechanisms toward the activated forms of gelatinases (matrix metalloproteinases 2 and 9). Specifically, we immunized mice with a synthetic molecule that mimics the conserved structure of the metalloenzyme catalytic zinc-histidine complex residing within the enzyme active site. This immunization procedure yielded selective function-blocking monoclonal antibodies directed against the catalytic zinc-protein complex and enzyme surface conformational epitopes of endogenous gelatinases. The therapeutic potential of these antibodies has been demonstrated with relevant mouse models of inflammatory bowel disease. Here we propose a general experimental strategy for generating inhibitory antibodies that effectively target the in vivo activity of dysregulated metalloproteinases by mimicking the mechanism employed by TIMPs.
- Department of Structural Biology, Weizmann Institute of Science, Rehovot, Israel.
Organizational Affiliation: