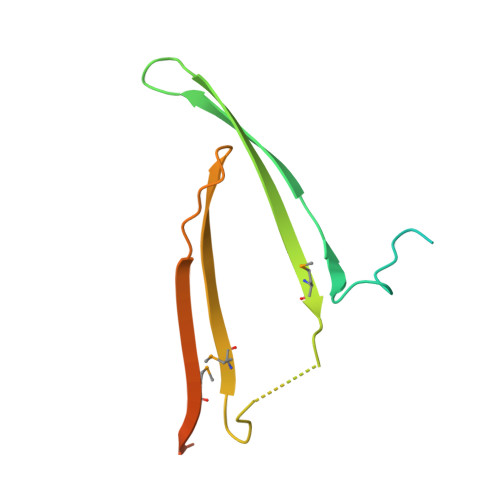
N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease.
He, Y.X., Zhang, N.N., Li, W.F., Jia, N., Chen, B.Y., Zhou, K., Zhang, J., Chen, Y., Zhou, C.Z.(2012) J Mol Biology 418: 197-207
- PubMed: 22387468
- DOI: https://doi.org/10.1016/j.jmb.2012.02.040
- Primary Citation of Related Structures:
3UA0 - PubMed Abstract:
Fibroins serve as the major building blocks of silk fiber. As the major component of fibroin, the fibroin heavy chain is a considerably large protein comprising N-terminal and C-terminal hydrophilic domains and 12 highly repetitive Gly-Ala-rich regions flanked by internal hydrophilic blocks. Here, we show the crystal structure of the fibroin N-terminal domain (FibNT) at pH 4.7, revealing a remarkable double-layered anti-parallel β-sheet with each layer comprising two FibNT molecules entangled together. We also show that FibNT undergoes a pH-responsive conformational transition from random coil to β-sheets at around pH 6.0. Dynamic light scattering demonstrates that FibNT tends to oligomerize as pH decreases to 6.0, and electron microscopy reveals micelle-like oligomers. Our results are consistent with the micelle assembly model of silk fibroin and, more importantly, show that the N-terminal domain in itself has the capacity to form micelle-like structures in response to pH decrease. Structural and mutagenesis analyses further reveal the important role of conserved acidic residues clustered in FibNT, such as Glu56 and Asp100, in preventing premature β-sheet formation at neutral pH. Collectively, we suggest that FibNT functions as a pH-responsive self-assembly module that could prevent premature β-sheet formation at neutral pH yet could initiate fibroin assembly as pH decreases along the lumen of the posterior silk gland to the anterior silk gland.
- Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230027, People's Republic of China.
Organizational Affiliation: