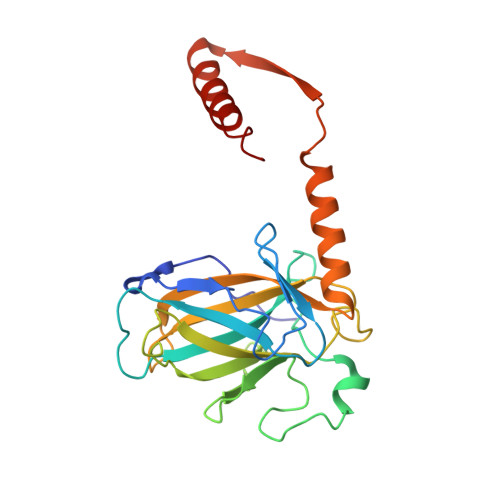
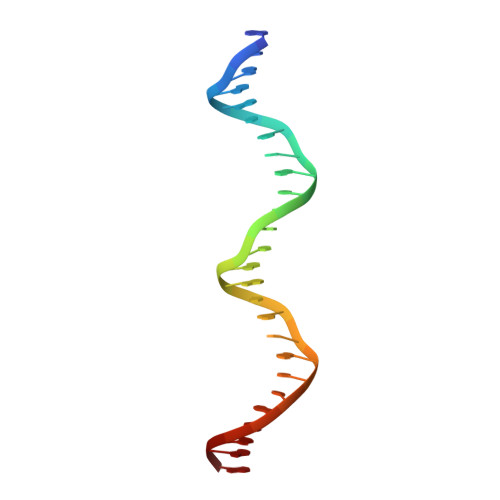
Crystal Structure of a Multidomain Human p53 Tetramer Bound to the Natural CDKN1A (p21) p53-Response Element.
Emamzadah, S., Tropia, L., Halazonetis, T.D.(2011) Mol Cancer Res 9: 1493-1499
- PubMed: 21933903
- DOI: https://doi.org/10.1158/1541-7786.MCR-11-0351
- Primary Citation of Related Structures:
3TS8 - PubMed Abstract:
The p53 tumor suppressor protein is a sequence-specific DNA-binding transcription factor. Structures of p53 bound to DNA have been described, but, so far, no structure has been determined of p53 bound to a natural p53-response element. We describe here the structure of a human p53 homotetramer encompassing both the DNA-binding and homo-oligomerization domains in complex with the natural p53-response element present upstream of the promoter of the CDKN1A (p21) gene. Similar to our previously described structures of human p53 tetramers bound to an artificial consensus DNA site, p53 DNA binding proceeds via an induced fit mechanism with loops L1 of two subunits adopting recessed conformations. Interestingly, the conformational change involving loop L1 is even more extreme than the one previously observed with the artificial consensus DNA site. In fact, the previously determined loop L1 conformation seems to be a transition intermediate between the non-DNA-bound and CDKN1A-bound states. Thus, the new structure further supports our model that recognition of specific DNA by p53 is associated with conformational changes within the DNA-binding domain of p53.
- Department of Molecular Biology, University of Geneva, Geneva, Switzerland.
Organizational Affiliation: