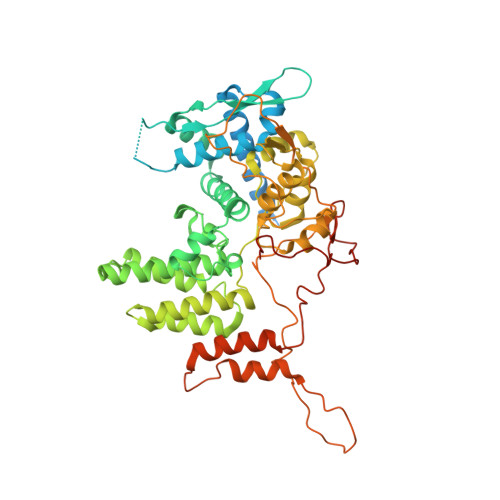
Structural basis for RNA binding and homo-oligomer formation by influenza B virus nucleoprotein
Ng, A.K.L., Lam, M.K.H., Zhang, H., Liu, J., Au, S.W.N., Chan, P.K.S., Wang, J., Shaw, P.C.(2012) J Virol 86: 6758-6767
- PubMed: 22496219
- DOI: https://doi.org/10.1128/JVI.00073-12
- Primary Citation of Related Structures:
3TJ0 - PubMed Abstract:
Influenza virus nucleoprotein (NP) is the major component of the viral ribonucleoprotein complex, which is crucial for the transcription and replication of the viral genome. We have determined the crystal structure of influenza B virus NP to a resolution of 3.2 Å. Influenza B NP contains a head, a body domain, and a tail loop. The electropositive groove between the head and body domains of influenza B NP is crucial for RNA binding. This groove also contains an extended flexible charged loop (amino acids [aa] 125 to 149), and two lysine clusters at the first half of this loop were shown to be crucial for binding RNA. Influenza B virus NP forms a crystallographic homotetramer by inserting the tail loop into the body domain of the neighboring NP molecule. A deeply buried salt bridge between R472 and E395 and a hydrophobic cluster at F468 are the major driving forces for the insertion. The analysis of the influenza B virus NP structure and function and comparisons with influenza A virus NP provide insights into the mechanisms of action and underpin efforts to design inhibitors for this class of proteins.
- Centre for Protein Science and Crystallography, School of Life Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Organizational Affiliation: