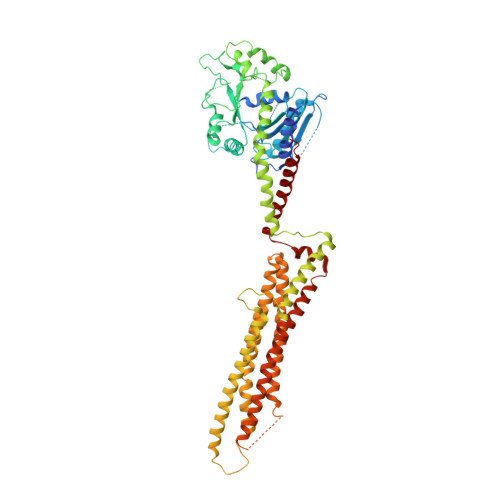
Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function.
Gao, S., von der Malsburg, A., Dick, A., Faelber, K., Schroder, G.F., Haller, O., Kochs, G., Daumke, O.(2011) Immunity 35: 514-525
- PubMed: 21962493
- DOI: https://doi.org/10.1016/j.immuni.2011.07.012
- Primary Citation of Related Structures:
3SZR - PubMed Abstract:
Human myxovirus resistance protein 1 (MxA) is an interferon-induced dynamin-like GTPase that acts as a cell-autonomous host restriction factor against many viral pathogens including influenza viruses. To study the molecular principles of its antiviral activity, we determined the crystal structure of nucleotide-free MxA, which showed an extended three-domain architecture. The central bundle signaling element (BSE) connected the amino-terminal GTPase domain with the stalk via two hinge regions. MxA oligomerized in the crystal via the stalk and the BSE, which in turn interacted with the stalk of the neighboring monomer. We demonstrated that the intra- and intermolecular domain interplay between the BSE and stalk was essential for oligomerization and the antiviral function of MxA. Based on these results, we propose a structural model for the mechano-chemical coupling in ring-like MxA oligomers as the principle mechanism for this unique antiviral effector protein.
- Max-Delbrück-Centrum for Molecular Medicine, Crystallography, Robert-Rössle-Strasse 10, 13125 Berlin, Germany. song.gao@mdc-berlin.de
Organizational Affiliation: