Structural and functional studies of casein kinase I-like protein from rice
Park, Y.I., Do, K.H., Kim, I.S., Park, H.H.(2012) Plant Cell Physiol 53: 304-311
- PubMed: 22199373
- DOI: https://doi.org/10.1093/pcp/pcr175
- Primary Citation of Related Structures:
3SV0 - PubMed Abstract:
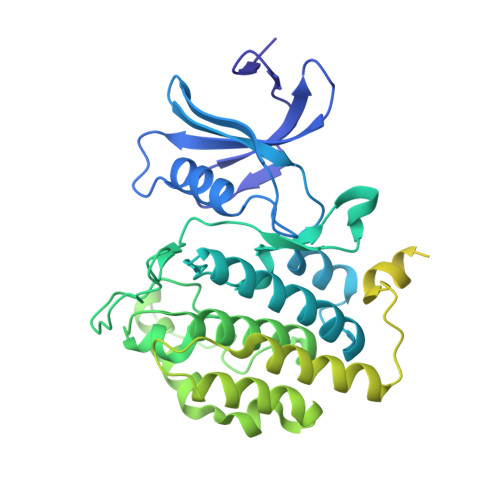
Casein kinase I (CKI) is a protein serine/threonine kinase that is highly conserved from plants to animals. It performs various functions in both the cytoplasm and nucleus, such as DNA repair, cell cycle, cytokinesis, vesicular trafficking, morphogenesis and circadian rhythm. CKI proteins contain a highly conserved kinase domain responsible for catalytic activity at the N-terminus and a highly diverse regulatory domain responsible for determining substrate specificity at the C-terminus. CKI-like protein has been identified in plants, including in rice, but its function and structure have not been reported. Here, we report the 2.0 Å crystal structure of the kinase domain of CKI-like protein from rice. Although the structure adopts the typical bi-lobal kinase architecture, the length and orientation of the glycine-rich ATP-binding motif are dynamic within the CKI family. A loop between α5 and α6 (the α5-α6 loop), which was previously not detected in the CKI family because of flexibility, was clearly detected in our structure. In addition, we identified a lipase as a substrate of CKI-like protein from rice. Phosphorylation of the lipase dramatically reduced its catalytic activity, suggesting that CKI may play a role in the regulation of lipase activity.
- School of Life Science and Biotechnology at Kyungpook National University, Daegu, South Korea.
Organizational Affiliation: