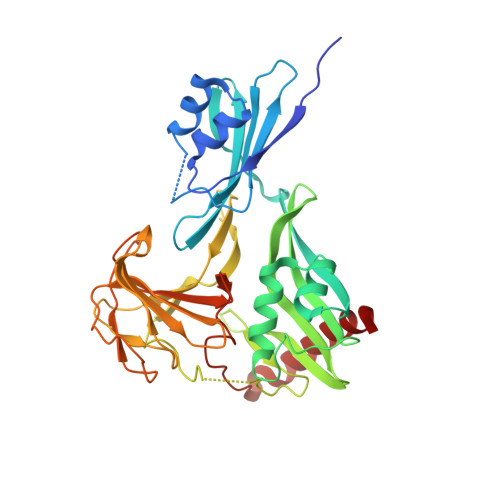
Crystal structure of outer membrane protein NMB0315 from Neisseria meningitidis.
Wang, X., Yang, X., Yang, C., Wu, Z., Xu, H., Shen, Y.(2011) PLoS One 6: e26845-e26845
- PubMed: 22046377
- DOI: https://doi.org/10.1371/journal.pone.0026845
- Primary Citation of Related Structures:
3SLU - PubMed Abstract:
NMB0315 is an outer membrane protein of Neisseria meningitidis serogroup B (NMB) and a potential candidate for a broad-spectrum vaccine against meningococcal disease. The crystal structure of NMB0315 was solved by single-wavelength anomalous dispersion (SAD) at a resolution of 2.4 Å and revealed to be a lysostaphin-type peptidase of the M23 metallopeptidase family. The overall structure consists of three well-separated domains and has no similarity to any previously published structure. However, only the topology of the carboxyl-terminal domain is highly conserved among members of this family, and this domain is a zinc-dependent catalytic unit. The amino-terminal domain of the structure blocks the substrate binding pocket in the carboxyl-terminal domain, indicating that the wild-type full-length protein is in an inactive conformational state. Our studies improve the understanding of the catalytic mechanism of M23 metallopeptidases.
- State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China.
Organizational Affiliation: