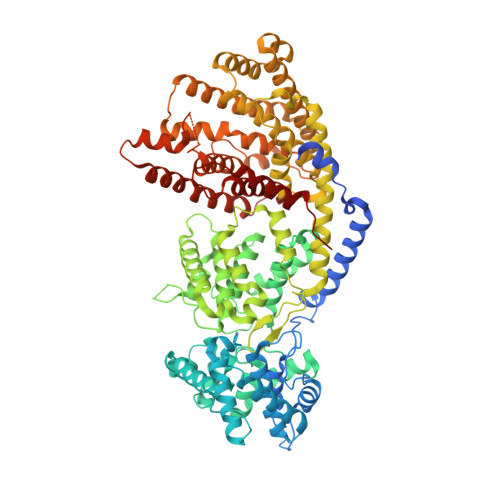
Structure of a three-domain sesquiterpene synthase: a prospective target for advanced biofuels production.
McAndrew, R.P., Peralta-Yahya, P.P., DeGiovanni, A., Pereira, J.H., Hadi, M.Z., Keasling, J.D., Adams, P.D.(2011) Structure 19: 1876-1884
- PubMed: 22153510
- DOI: https://doi.org/10.1016/j.str.2011.09.013
- Primary Citation of Related Structures:
3SAE, 3SDQ, 3SDR, 3SDT, 3SDU, 3SDV - PubMed Abstract:
The sesquiterpene bisabolene was recently identified as a biosynthetic precursor to bisabolane, an advanced biofuel with physicochemical properties similar to those of D2 diesel. High-titer microbial bisabolene production was achieved using Abies grandis α-bisabolene synthase (AgBIS). Here, we report the structure of AgBIS, a three-domain plant sesquiterpene synthase, crystallized in its apo form and bound to five different inhibitors. Structural and biochemical characterization of the AgBIS terpene synthase Class I active site leads us to propose a catalytic mechanism for the cyclization of farnesyl diphosphate into bisabolene via a bisabolyl cation intermediate. Further, we describe the nonfunctional AgBIS Class II active site whose high similarity to bifunctional diterpene synthases makes it an important link in understanding terpene synthase evolution. Practically, the AgBIS crystal structure is important in future protein engineering efforts to increase the microbial production of bisabolene.
- Joint BioEnergy Institute, 5885 Hollis Avenue, Emeryville, CA 94608, USA.
Organizational Affiliation: