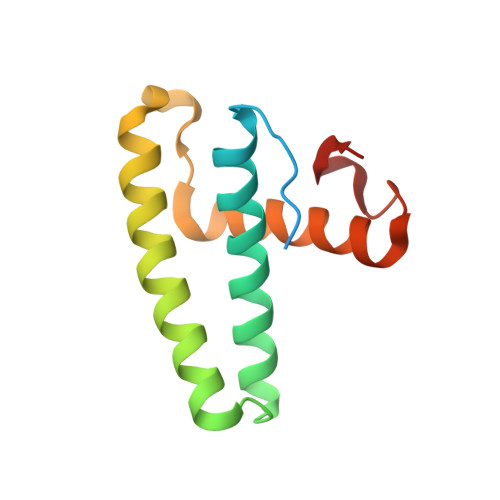
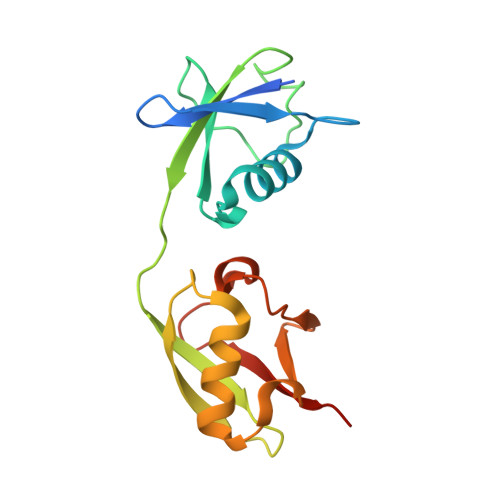
Structural basis for the sequence-specific recognition of human ISG15 by the NS1 protein of influenza B virus.
Guan, R., Ma, L.C., Leonard, P.G., Amer, B.R., Sridharan, H., Zhao, C., Krug, R.M., Montelione, G.T.(2011) Proc Natl Acad Sci U S A 108: 13468-13473
- PubMed: 21808041
- DOI: https://doi.org/10.1073/pnas.1107032108
- Primary Citation of Related Structures:
3SDL - PubMed Abstract:
Interferon-induced ISG15 conjugation plays an important antiviral role against several viruses, including influenza viruses. The NS1 protein of influenza B virus (NS1B) specifically binds only human and nonhuman primate ISG15s and inhibits their conjugation. To elucidate the structural basis for the sequence-specific recognition of human ISG15, we determined the crystal structure of the complex formed between human ISG15 and the N-terminal region of NS1B (NS1B-NTR). The NS1B-NTR homodimer interacts with two ISG15 molecules in the crystal and also in solution. The two ISG15-binding sites on the NS1B-NTR dimer are composed of residues from both chains, namely residues in the RNA-binding domain (RBD) from one chain, and residues in the linker between the RBD and the effector domain from the other chain. The primary contact region of NS1B-NTR on ISG15 is composed of residues at the junction of the N-terminal ubiquitin-like (Ubl) domain and the short linker region between the two Ubl domains, explaining why the sequence of the short linker in human and nonhuman primate ISG15s is essential for the species-specific binding of these ISG15s. In addition, the crystal structure identifies NS1B-NTR binding sites in the N-terminal Ubl domain of ISG15, and shows that there are essentially no contacts with the C-terminal Ubl domain of ISG15. Consequently, NS1B-NTR binding to ISG15 would not occlude access of the C-terminal Ubl domain of ISG15 to its conjugating enzymes. Nonetheless, transfection assays show that NS1B-NTR binding of ISG15 is responsible for the inhibition of interferon-induced ISG15 conjugation in cells.
- Center for Advanced Biotechnology and Medicine, Rutgers University, Piscataway NJ 08854, USA.
Organizational Affiliation: