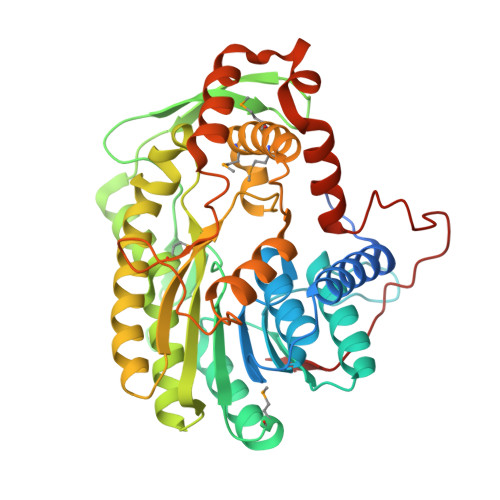
Determination of the Crystal Structure and Active Residues of FabV, the Enoyl-ACP Reductase from Xanthomonas oryzae.
Li, H., Zhang, X., Bi, L., He, J., Jiang, T.(2011) PLoS One 6: e26743-e26743
- PubMed: 22039545
- DOI: https://doi.org/10.1371/journal.pone.0026743
- Primary Citation of Related Structures:
3S8M - PubMed Abstract:
Enoyl-ACP reductase (ENR) catalyses the last reduction reaction in the fatty acid elongation cycle in bacteria and is a good antimicrobial target candidate. FabV is the most recently discovered class of ENR, but we lack information about the atomic structure and the key residues involved in reductase activity except for the known conserved tyrosine and lysine residues in the Y-X(8)-K active site motif. Here we report the crystal structure of FabV from Xanthomonas oryzae (xoFabV). The crystal structure of this enzyme has been solved to 1.6 Å resolution in space group P2(1)2(1)2(1). The model of xoFabV consists of one monomer in the asymmetric unit which is composed of 13 α-helices and 11 β-strands, representing a canonical Rossmann fold architecture. Structural comparison presents that the locations of the conserved tyrosine (Y236) and lysine (K245) residues in the Y-X(8)-K active site motif of xoFabV and the Y-X(6)-K motif of ecFabI are notably similar. However, the conformations of Y236 in xoFabV and Y156 in ecFabI are distinct. Structure-based site-directed mutagenesis and enzymatic activity assays reveal that in addition to the conserved Y236 and K245 in the Y-X(8)-K motif, Y53, D111 and Y226 are key residues implicated in the reductase activity, and F113 and T276 are also important for enzyme function. Moreover, a proposed active lysine located immediately after the Y-X(8)-K motif in FabV from Burkholderia mallei (bmFabV) is altered to an inactive V246 in xoFabV. We determine the first crystal structure of the FabV enzyme and identify several residues important for its enzymatic activity. These findings lay a solid foundation for the development of specific antibacterial inhibitors of the pathogenic bacteria, such as Vibrio cholerae, Burkholderia species and Xanthomonas species.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, People's Republic of China.
Organizational Affiliation: