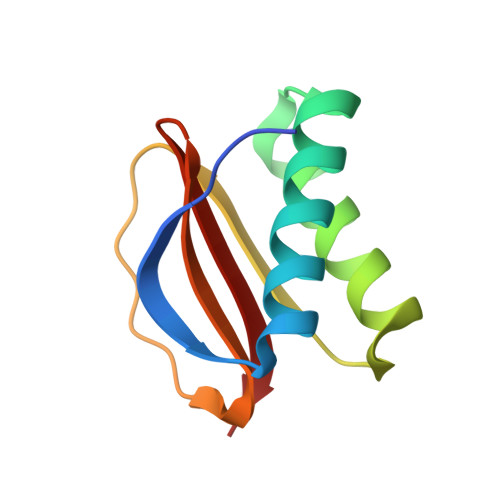
Dynein light chain 8a of Toxoplasma gondii, a unique conoid-localized beta-strand-swapped homodimer, is required for an efficient parasite growth.
Qureshi, B.M., Hofmann, N.E., Arroyo-Olarte, R.D., Nickl, B., Hoehne, W., Jungblut, P.R., Lucius, R., Scheerer, P., Gupta, N.(2013) FASEB J 27: 1034-1047
- PubMed: 23233536
- DOI: https://doi.org/10.1096/fj.11-180992
- Primary Citation of Related Structures:
3RJS - PubMed Abstract:
Dynein light chain 8 (DLC8) is a ubiquitous eukaryotic protein regulating diverse cellular functions. We show that the obligate intracellular parasite Toxoplasma gondii harbors 4 DLC8 proteins (TgDLC8a-d), of which only TgDLC8a clusters in the mainstream LC8 class. TgDLC8b-d proteins form a divergent and alveolate-specific clade. TgDLC8b-d proteins are largely cytosolic, whereas TgDLC8a resides in the conoid at the apical end of T. gondii. The apical location of TgDLC8a is also not shared by its nearly identical Eimeria (EtDLC8a), Plasmodium (PfDLC8), or human (HsDLC8) orthologs. Notwithstanding an exclusive conoid targeting, TgDLC8a exhibits a classical LC8 structure. It forms a homodimer by swapping of the β strands that interact with the antiparallel β' strands of the opposing monomers. The TgDLC8a dimer contains two identical binding grooves and appears to be adapted for multitarget recognition. By contrast, the previously reported PfDLC8 homodimer is shaped by binding of the β strand with the parallel β' strand and lacks such a distinct binding interface. Our comparisons suggest an unexpected structural and functional divergence of the two otherwise conserved proteins from apicomplexan parasites. Finally, we demonstrate that a phosphomimetic S88E mutation renders the TgDLC8a-S88E mutant monomeric and cytosolic in T. gondii, and its overexpression inhibits the parasite growth in human fibroblasts.
- Department of Molecular Parasitology, Humboldt University, Berlin, D-10115 Germany.
Organizational Affiliation: