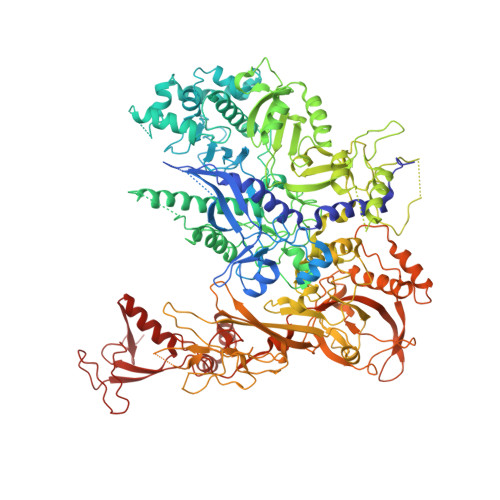
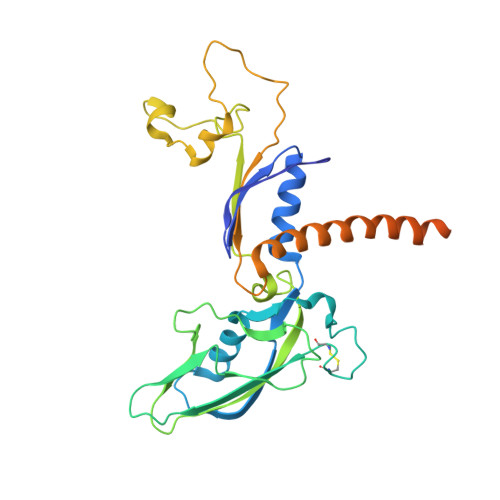
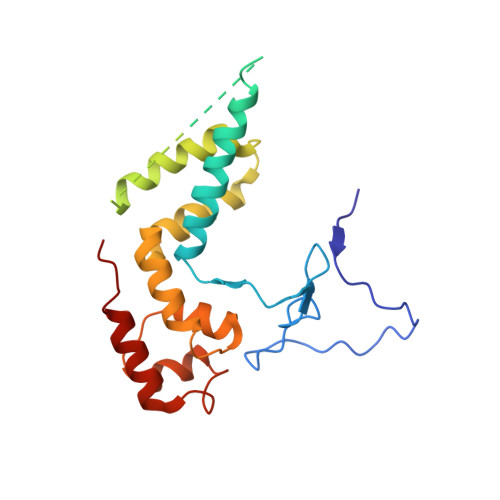
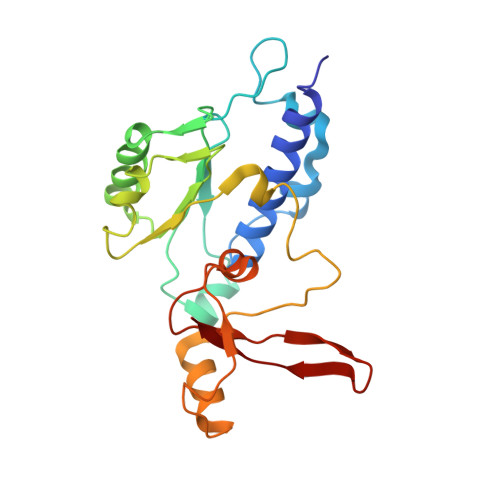
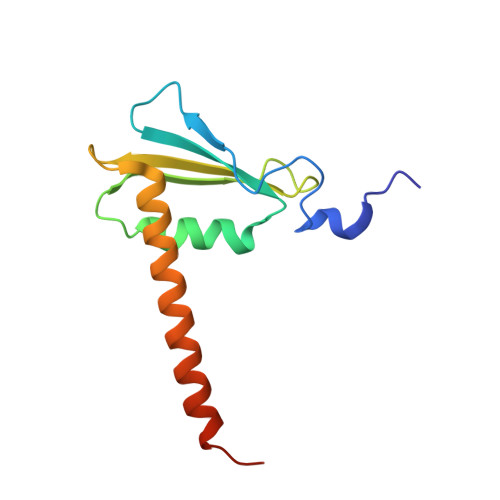
Evolution of two modes of intrinsic RNA polymerase transcript cleavage.
Ruan, W., Lehmann, E., Thomm, M., Kostrewa, D., Cramer, P.(2011) J Biological Chem 286: 18701-18707
- PubMed: 21454497
- DOI: https://doi.org/10.1074/jbc.M111.222273
- Primary Citation of Related Structures:
3QT1 - PubMed Abstract:
During gene transcription, the RNA polymerase (Pol) active center can catalyze RNA cleavage. This intrinsic cleavage activity is strong for Pol I and Pol III but very weak for Pol II. The reason for this difference is unclear because the active centers of the polymerases are virtually identical. Here we show that Pol II gains strong cleavage activity when the C-terminal zinc ribbon domain (C-ribbon) of subunit Rpb9 is replaced by its counterpart from the Pol III subunit C11. X-ray analysis shows that the C-ribbon has detached from its site on the Pol II surface and is mobile. Mutagenesis indicates that the C-ribbon transiently inserts into the Pol II pore to complement the active center. This mechanism is also used by transcription factor IIS, a factor that can bind Pol II and induce strong RNA cleavage. Together with published data, our results indicate that Pol I and Pol III contain catalytic C-ribbons that complement the active center, whereas Pol II contains a non-catalytic C-ribbon that is immobilized on the enzyme surface. Evolution of the Pol II system may have rendered mRNA transcript cleavage controllable by the dissociable factor transcription factor IIS to enable promoter-proximal gene regulation and elaborate 3'-processing and transcription termination.
- Gene Center and Department of Biochemistry, Center for Integrated Protein Science Munich, Ludwig-Maximilians-Universität München, Feodor-Lynen-Strasse 25, 81377 Munich, Germany.
Organizational Affiliation: