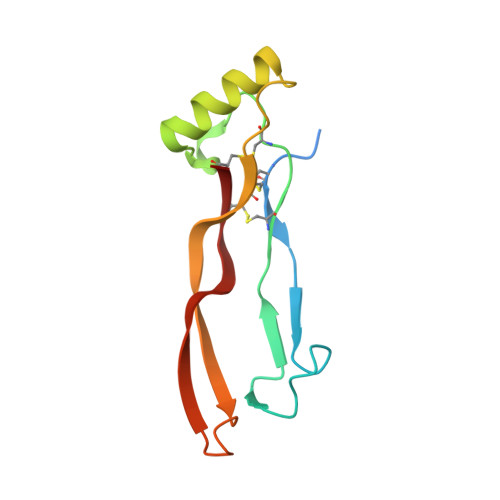
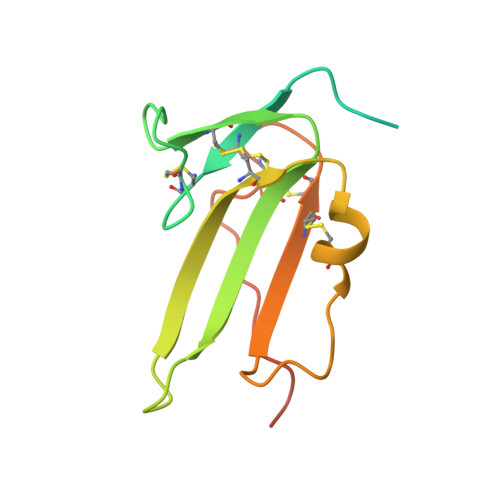
GDF-5 can act as a context-dependent BMP-2 antagonist.
Klammert, U., Mueller, T.D., Hellmann, T.V., Wuerzler, K.K., Kotzsch, A., Schliermann, A., Schmitz, W., Kuebler, A.C., Sebald, W., Nickel, J.(2015) BMC Biol 13: 77-77
- PubMed: 26385096
- DOI: https://doi.org/10.1186/s12915-015-0183-8
- Primary Citation of Related Structures:
3QB4 - PubMed Abstract:
Bone morphogenetic protein (BMP)-2 and growth and differentiation factor (GDF)-5 are two related transforming growth factor (TGF)-β family members with important functions in embryonic development and tissue homeostasis. BMP-2 is best known for its osteoinductive properties whereas GDF-5-as evident from its alternative name, cartilage derived morphogenetic protein 1-plays an important role in the formation of cartilage. In spite of these differences both factors signal by binding to the same subset of BMP receptors, raising the question how these different functionalities are generated. The largest difference in receptor binding is observed in the interaction with the type I receptor BMPR-IA. GDF-5, in contrast to BMP-2, shows preferential binding to the isoform BMPR-IB, which is abrogated by a single amino acid (A57R) substitution. The resulting variant, GDF-5 R57A, represents a "BMP-2 mimic" with respect to BMP receptor binding. In this study we thus wanted to analyze whether the two growth factors can induce distinct signals via an identically composed receptor. Unexpectedly and dependent on the cellular context, GDF-5 R57A showed clear differences in its activity compared to BMP-2. In ATDC-5 cells, both ligands induced alkaline phosphatase (ALP) expression with similar potency. But in C2C12 cells, the BMP-2 mimic GDF-5 R57A (and also wild-type GDF-5) clearly antagonized BMP-2-mediated ALP expression, despite signaling in both cell lines occurring solely via BMPR-IA. The BMP-2- antagonizing properties of GDF-5 and GDF-5 R57A could also be observed in vivo when implanting BMP-2 and either one of the two GDF-5 ligands simultaneously at heterotopic sites. Although comparison of the crystal structures of the GDF-5 R57A:BMPR-IAEC- and BMP-2:BMPR-IAEC complex revealed small ligand-specific differences, these cannot account for the different signaling characteristics because the complexes seem identical in both differently reacting cell lines. We thus predict an additional component, most likely a not yet identified GDF-5-specific co-receptor, which alters the output of the signaling complexes. Hence the presence or absence of this component then switches GDF-5's signaling capabilities to act either similar to BMP-2 or as a BMP-2 antagonist. These findings might shed new light on the role of GDF-5, e.g., in cartilage maintenance and/or limb development in that it might act as an inhibitor of signaling events initiated by other BMPs.
- Lehrstuhl für Mund-, Kiefer- und plastische Gesichtschirurgie, Universitätsklinikum Würzburg, Pleicherwall 2, 97070, Würzburg, Germany. Klammert_U@klinik.uni-wuerzburg.de.
Organizational Affiliation: