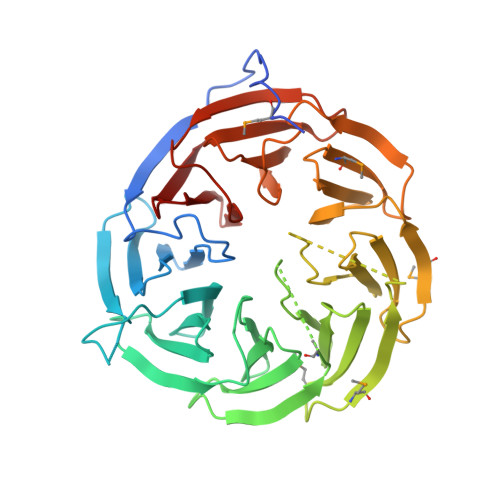
The Crystal Structure of BamB Suggests Interactions with BamA and Its Role within the BAM Complex.
Noinaj, N., Fairman, J.W., Buchanan, S.K.(2011) J Mol Biology 407: 248-260
- PubMed: 21277859
- DOI: https://doi.org/10.1016/j.jmb.2011.01.042
- Primary Citation of Related Structures:
3Q7M, 3Q7N, 3Q7O - PubMed Abstract:
Escherichia coli BamB is the largest of four lipoproteins in the β-barrel assembly machinery (BAM) complex. It interacts with the periplasmic domain of BamA, an integral outer membrane protein (OMP) essential for OMP biogenesis. Although BamB is not essential, it serves an important function in the BAM complex, significantly increasing the folding efficiency of some OMPs in vivo and in vitro. To learn more about the BAM complex, we solved structures of BamB in three different crystal forms. BamB crystallized in space groups P2(1)3, I222, and P2(1)2(1)2(1), with one molecule per asymmetric unit in each case. Crystals from the space group I222 diffracted to 1. 65-Å resolution. BamB forms an eight-bladed β-propeller with a central pore and is shaped like a doughnut. A DALI search revealed that BamB shares structural homology to several eukaryotic proteins containing WD40 repeat domains, which commonly have β-propeller folds and often serve as scaffolding proteins within larger multi-protein complexes that carry out signal transduction, cell division, and chemotaxis. Using mutagenesis data from previous studies, we docked BamB onto a BamA structural model and assessed known and possible interactions between these two proteins. Our data suggest that BamB serves as a scaffolding protein within the BAM complex by optimally orienting the flexible periplasmic domain of BamA for interaction with other BAM components and chaperones. This may facilitate integration of newly synthesized OMPs into the outer membrane.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-8030, USA.
Organizational Affiliation: