Crystal structure of T2-depleted fungal laccase from Trametes hirsuta at low and high dose of ionization radiation.
Polyakov, K.M., Fedorova, T.V., Kurzeev, S.A., Popov, A.N., Lamzin, V.S., Koroleva, O.V.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Laccase | 499 | Trametes hirsuta | Mutation(s): 0 EC: 1.10.3.2 | 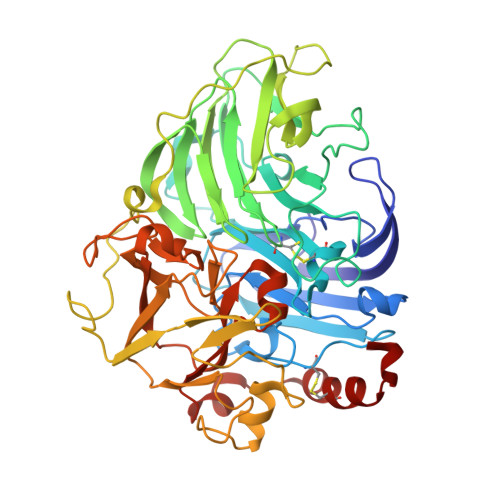 | |
UniProt | |||||
Find proteins for B2L9C1 (Trametes hirsuta) Explore B2L9C1 Go to UniProtKB: B2L9C1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B2L9C1 | ||||
Glycosylation | |||||
| Glycosylation Sites: 4 | |||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | B | 7 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G55220VL GlyCosmos: G55220VL GlyGen: G55220VL | |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | I [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| CU Query on CU | E [auth A], F [auth A], G [auth A], H [auth A] | COPPER (II) ION Cu JPVYNHNXODAKFH-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 52.653 | α = 90 |
| b = 77.051 | β = 90 |
| c = 130.216 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| MOLREP | phasing |
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |