Structural basis of binding by cyclic nonphosphorylated Peptide antagonists of grb7 implicated in breast cancer progression
Ambaye, N.D., Pero, S.C., Gunzburg, M.J., Yap, M., Clayton, D.J., Del Borgo, M.P., Perlmutter, P., Aguilar, M.I., Shukla, G.S., Peletskaya, E., Cookson, M.M., Krag, D.N., Wilce, M.C., Wilce, J.A.(2011) J Mol Biology 412: 397-411
- PubMed: 21802427
- DOI: https://doi.org/10.1016/j.jmb.2011.07.030
- Primary Citation of Related Structures:
3PQZ - PubMed Abstract:
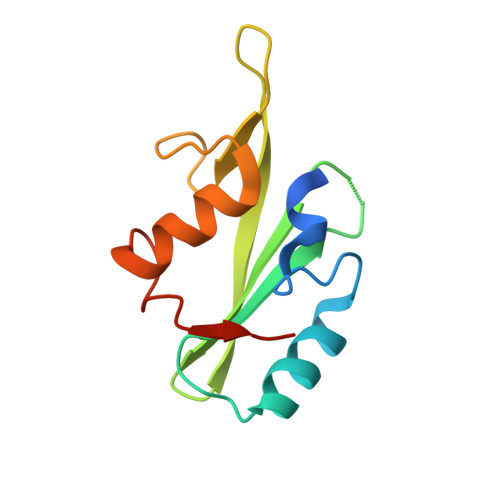
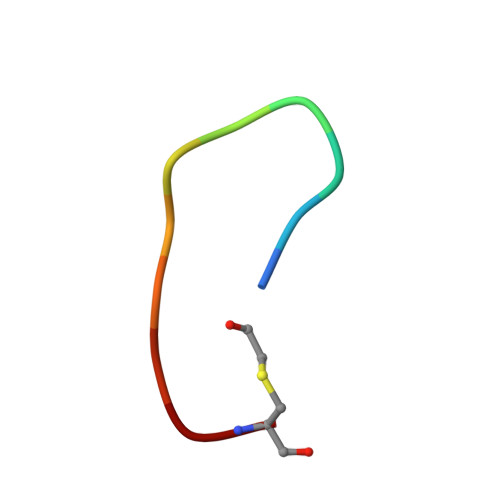
Growth-receptor-bound protein (Grb)7 is an adapter protein aberrantly overexpressed, along with the erbB-2 receptor in breast cancer and in other cancers. Normally recruited to focal adhesions with a role in cell migration, it is associated with erbB-2 in cancer cells and is found to exacerbate cancer progression via stimulation of cell migration and proliferation. The G7-18NATE peptide (sequence: WFEGYDNTFPC cyclized via a thioether bond) is a nonphosphorylated peptide that was developed for the specific inhibition of Grb7 by blocking its SH2 domain. Cell-permeable versions of G7-18NATE are effective in the reduction of migration and proliferation in Grb7-overexpressing cells. It thus represents a promising starting point for the development of a therapeutic against Grb7. Here, we report the crystal structure of the G7-18NATE peptide in complex with the Grb7-SH2 domain, revealing the structural basis for its interaction. We also report further rounds of phage display that have identified G7-18NATE analogues with micromolar affinity for Grb7-SH2. These peptides retained amino acids F2, G4, and F9, as well as the YDN motif that the structural biology study showed to be the main residues in contact with the Grb7-SH2 domain. Isothermal titration calorimetry measurements reveal similar and better binding affinity of these peptides compared with G7-18NATE. Together, this study facilitates the optimization of second-generation inhibitors of Grb7.
- Department of Biochemistry and Molecular Biology, Monash University, Wellington Road, VIC 3800, Australia.
Organizational Affiliation: