Structure-based design and synthesis of cell active N-pyrazole, N-thiazole urea inhibitors of the MAP kinase p38alpha
Getlik, M., Gruetter, C., Simard, J.R., Van Otterlo, W., Robubi, A., Aust, B., Rauh, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mitogen-activated protein kinase 14 | 360 | Homo sapiens | Mutation(s): 4 Gene Names: MAPK14, CSBP, CSBP1, CSBP2, CSPB1, MXI2 EC: 2.7.11.24 | 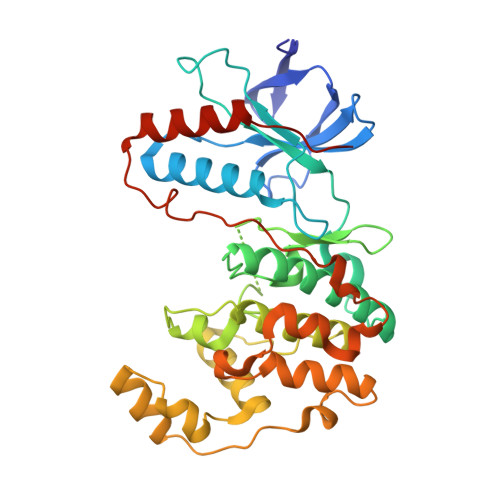 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q16539 (Homo sapiens) Explore Q16539 Go to UniProtKB: Q16539 | |||||
PHAROS: Q16539 GTEx: ENSG00000112062 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q16539 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| DG7 Query on DG7 | C [auth A] | 1-[3-tert-butyl-1-(4-methylphenyl)-1H-pyrazol-5-yl]-3-{4-[2-(pyridin-3-ylmethoxy)ethyl]-1,3-thiazol-2-yl}urea C26 H30 N6 O2 S FNKUETGWXHXSHJ-UHFFFAOYSA-N |  | ||
| BOG Query on BOG | B [auth A] | octyl beta-D-glucopyranoside C14 H28 O6 HEGSGKPQLMEBJL-RKQHYHRCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 69.11 | α = 90 |
| b = 69.97 | β = 90 |
| c = 74.53 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XSCALE | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data scaling |
| XDS | data reduction |