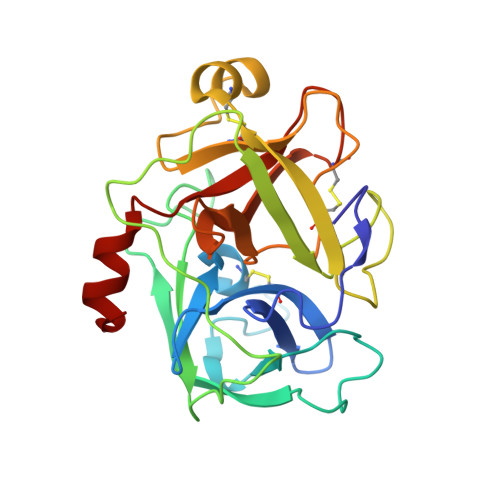
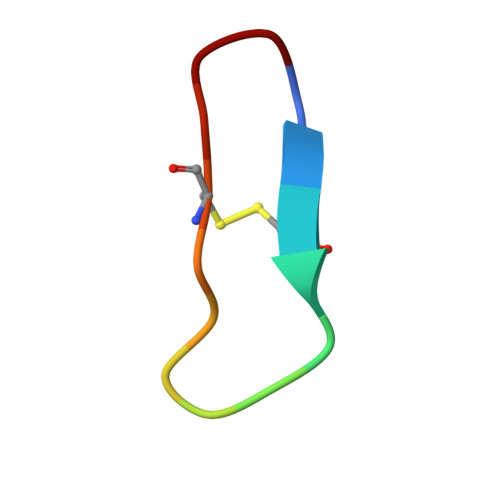
Structure of catalytic domain of Matriptase in complex with Sunflower trypsin inhibitor-1.
Yuan, C., Chen, L., Meehan, E.J., Daly, N., Craik, D.J., Huang, M., Ngo, J.C.(2011) BMC Struct Biol 11: 30-30
- PubMed: 21693064
- DOI: https://doi.org/10.1186/1472-6807-11-30
- Primary Citation of Related Structures:
3P8F, 3P8G - PubMed Abstract:
Matriptase is a type II transmembrane serine protease that is found on the surfaces of epithelial cells and certain cancer cells. Matriptase has been implicated in the degradation of certain extracellular matrix components as well as the activation of various cellular proteins and proteases, including hepatocyte growth factor and urokinase. Sunflower trypsin inhibitor-1 (SFTI-1), a cyclic peptide inhibitor originally isolated from sunflower seeds, exhibits potent inhibitory activity toward matriptase. We have engineered and produced recombinant proteins of the matriptase protease domain, and have determined the crystal structures of the protease:SFTI-1 complex at 2.0 Å as well as the protease:benzamidine complex at 1.2 Å. These structures elaborate the structural basis of substrate selectivity of matriptase, and show that the matriptase S1 substrate specificity pocket is larger enough to allow movement of benzamidine inside the S1 pocket. Our study also reveals that SFTI-1 binds to matriptase in a way similar to its binding to trypsin despite the significantly different isoelectric points of the two proteins (5.6 vs. 8.2). This work helps to define the structural basis of substrate specificity of matriptase and the interactions between the inhibitor and protease. The complex structure also provides a structural template for designing new SFTI-1 derivatives with better potency and selectivity against matriptase and other proteases.
- State Key Lab of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China.
Organizational Affiliation: