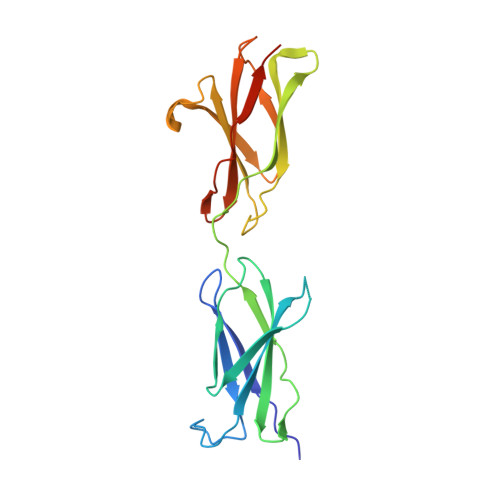
Crystal structure of a hemojuvelin-binding fragment of neogenin at 1.8A.
Yang, F., West, A.P., Bjorkman, P.J.(2011) J Struct Biol 174: 239-244
- PubMed: 20971194
- DOI: https://doi.org/10.1016/j.jsb.2010.10.005
- Primary Citation of Related Structures:
3P4L - PubMed Abstract:
Neogenin is a type I transmembrane glycoprotein with a large ectodomain containing tandem immunoglobulin-like and fibronectin type III (FNIII) domains. Closely related to the tumor suppressor gene DCC, neogenin functions in critical biological processes through binding to various ligands, including netrin, repulsive guidance molecules, and the iron regulatory protein hemojuvelin. We previously reported that neogenin binds to hemojuvelin through its membrane-proximal fifth and sixth FNIII domains (FN5-6), with domain 6 (FN6) contributing the majority of critical binding interactions. Here we present the crystal structure of FN5-6, the hemojuvelin-binding fragment of human neogenin, at 1.8Å. The two FNIII domains are orientated nearly linearly, a domain arrangement most similar to that of a tandem FNIII-containing fragment within the cytoplasmic tail of the β4 integrin. By mapping surface-exposed residues that differ between neogenin FN5-6 and the comparable domains from DCC, which does not bind hemojuvelin, we identified a potential hemojuvelin-binding site on neogenin FN6. Neogenin FN5, which does not bind hemojuvelin in isolation, exhibits a highly electropositive surface, which may be involved in interactions with negatively-charged polysaccharides or phospholipids in the membrane bilayer. The neogenin FN5-6 structure can be used to facilitate a molecular understanding of neogenin's interaction with hemojuvelin to regulate iron homeostasis and with hemojuvelin-related repulsive guidance molecules to mediate axon guidance.
- Graduate Option in Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA.
Organizational Affiliation: