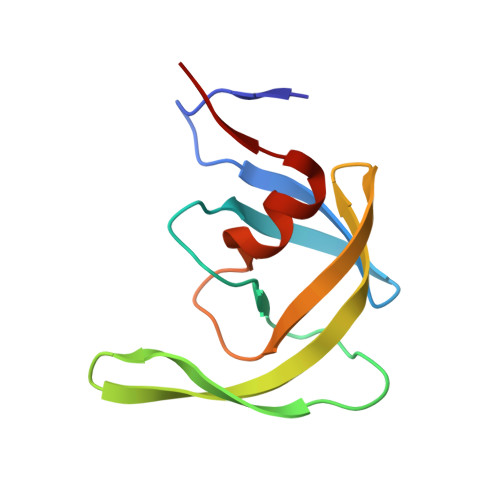
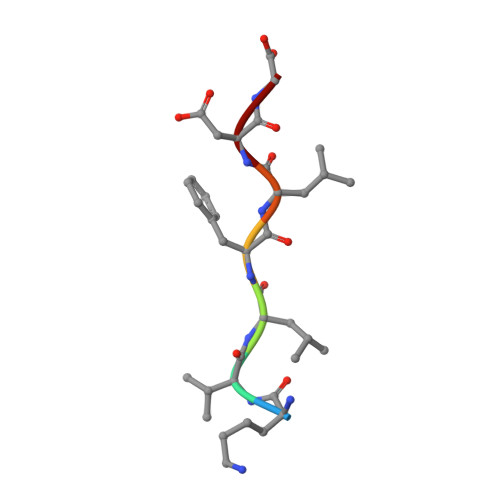
Nine Crystal Structures Determine the Substrate Envelope of the MDR HIV-1 Protease.
Liu, Z., Wang, Y., Brunzelle, J., Kovari, I.A., Kovari, L.C.(2011) Protein J 30: 173-183
- PubMed: 21394574
- DOI: https://doi.org/10.1007/s10930-011-9316-2
- Primary Citation of Related Structures:
3OTS, 3OTY, 3OU1, 3OU3, 3OU4, 3OUA, 3OUB, 3OUC, 3OUD - PubMed Abstract:
Under drug selection pressure, emerging mutations render HIV-1 protease drug resistant, leading to the therapy failure in anti-HIV treatment. It is known that nine substrate cleavage site peptides bind to wild type (WT) HIV-1 protease in a conserved pattern. However, how the multidrug-resistant (MDR) HIV-1 protease binds to the substrate cleavage site peptides is yet to be determined. MDR769 HIV-1 protease (resistant mutations at residues 10, 36, 46, 54, 62, 63, 71, 82, 84, and 90) was selected for present study to understand the binding to its natural substrates. MDR769 HIV-1 protease was co-crystallized with nine substrate cleavage site hepta-peptides. Crystallographic studies show that MDR769 HIV-1 protease has an expanded substrate envelope with wide open flaps. Furthermore, ligand binding energy calculations indicate weaker binding in MDR769 HIV-1 protease-substrate complexes. These results help in designing the next generation of HIV-1 protease inhibitors by targeting the MDR HIV-1 protease.
- Department of Biochemistry and Molecular Biology, Wayne State University, School of Medicine, 540 E. Canfield Avenue, 4263 Scott Hall, Detroit, MI 48201, USA.
Organizational Affiliation: