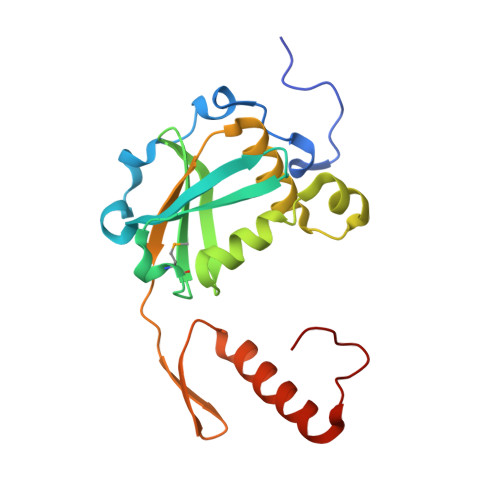
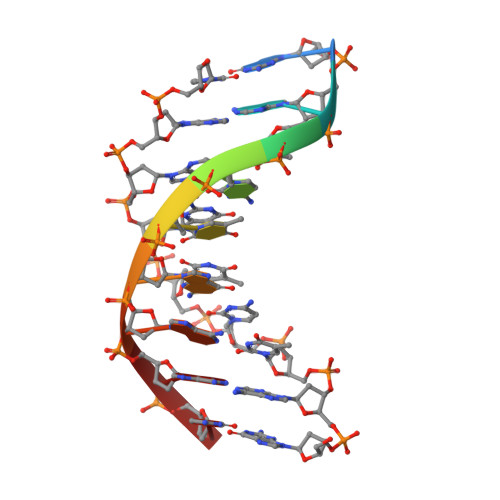
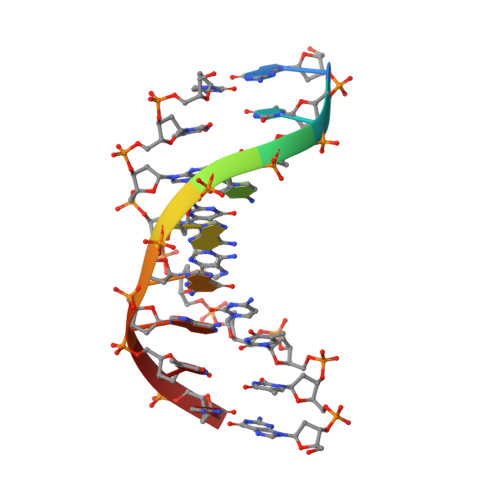
Hpy188I-DNA pre- and post-cleavage complexes--snapshots of the GIY-YIG nuclease mediated catalysis.
Sokolowska, M., Czapinska, H., Bochtler, M.(2011) Nucleic Acids Res 39: 1554-1564
- PubMed: 20935048
- DOI: https://doi.org/10.1093/nar/gkq821
- Primary Citation of Related Structures:
3OQG, 3OR3 - PubMed Abstract:
The GIY-YIG nuclease domain is present in all kingdoms of life and has diverse functions. It is found in the eukaryotic flap endonuclease and Holliday junction resolvase Slx1-Slx4, the prokaryotic nucleotide excision repair proteins UvrC and Cho, and in proteins of 'selfish' genetic elements. Here we present the structures of the ternary pre- and post-cleavage complexes of the type II GIY-YIG restriction endonuclease Hpy188I with DNA and a surrogate or catalytic metal ion, respectively. Our structures suggest that GIY-YIG nucleases catalyze DNA hydrolysis by a single substitution reaction. They are consistent with a previous proposal that a tyrosine residue (which we expect to occur in its phenolate form) acts as a general base for the attacking water molecule. In contrast to the earlier proposal, our data identify the general base with the GIY and not the YIG tyrosine. A conserved glutamate residue (Glu149 provided in trans in Hpy188I) anchors a single metal cation in the active site. This metal ion contacts the phosphate proS oxygen atom and the leaving group 3'-oxygen atom, presumably to facilitate its departure. Taken together, our data reveal striking analogy in the absence of homology between GIY-YIG and ββα-Me nucleases.
- International Institute of Molecular and Cell Biology, Trojdena 4, 02-109 Warsaw, Poland.
Organizational Affiliation: