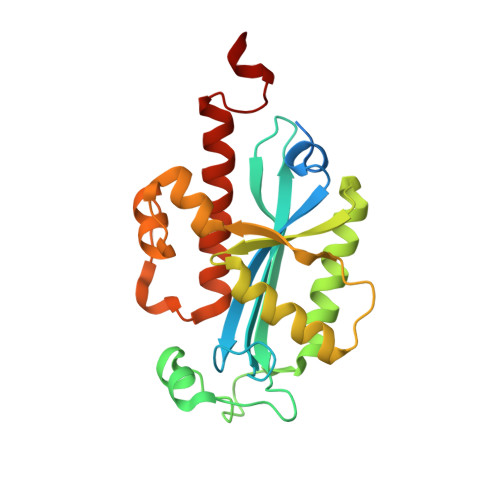
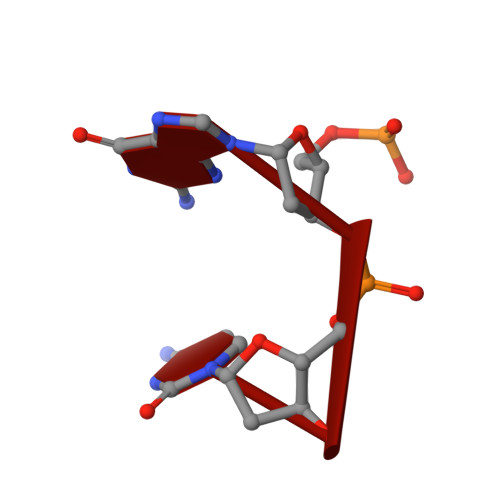
Structural basis for RNA trimming by RNase T in stable RNA 3'-end maturation
Hsiao, Y.-Y., Yang, C.-C., Lin, C.L., Lin, J.L.J., Duh, Y., Yuan, H.S.(2011) Nat Chem Biol 7: 236-243
- PubMed: 21317904
- DOI: https://doi.org/10.1038/nchembio.524
- Primary Citation of Related Structures:
3NGY, 3NGZ, 3NH0, 3NH1, 3NH2 - PubMed Abstract:
RNA maturation relies on various exonucleases to remove nucleotides successively from the 5' or 3' end of nucleic acids. However, little is known regarding the molecular basis for substrate and cleavage preference of exonucleases. Our biochemical and structural analyses on RNase T-DNA complexes show that the RNase T dimer has an ideal architecture for binding a duplex with a short 3' overhang to produce a digestion product of a duplex with a 2-nucleotide (nt) or 1-nt 3' overhang, depending on the composition of the last base pair in the duplex. A 'C-filter' in RNase T screens out the nucleic acids with 3'-terminal cytosines for hydrolysis by inducing a disruptive conformational change at the active site. Our results reveal the general principles and the working mechanism for the final trimming step made by RNase T in the maturation of stable RNA and pave the way for the understanding of other DEDD family exonucleases.
- Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu, Taiwan, ROC.
Organizational Affiliation: